Research | 2024-03-13
BackJingkai YANG | 03/12/2024
Organic small-molecule fluorophores have attracted increasing attention in biosensing, disease diagnostics, semiconductor material, and dye-sensitized solar cells due to their flexible chemical structure and adjustable optical performance. So far, although plenty of excellent organic small-molecule fluorescent dyes have been developed, the detection of these dyes’ signals still requires filters and optoelectronic equipment, limiting their application in scenarios with simple settings. This is due to the established dyes, designed mainly based on a few classical fluorophore motifs, tend to exhibit small Stokes shift (typically < 30 nm, such as rhodamine, fluorescein, boron dipyrromethene (BODIPY), cyanine) or show large Stokes shifts but low brightness (such as naphthalimide or coumarin).
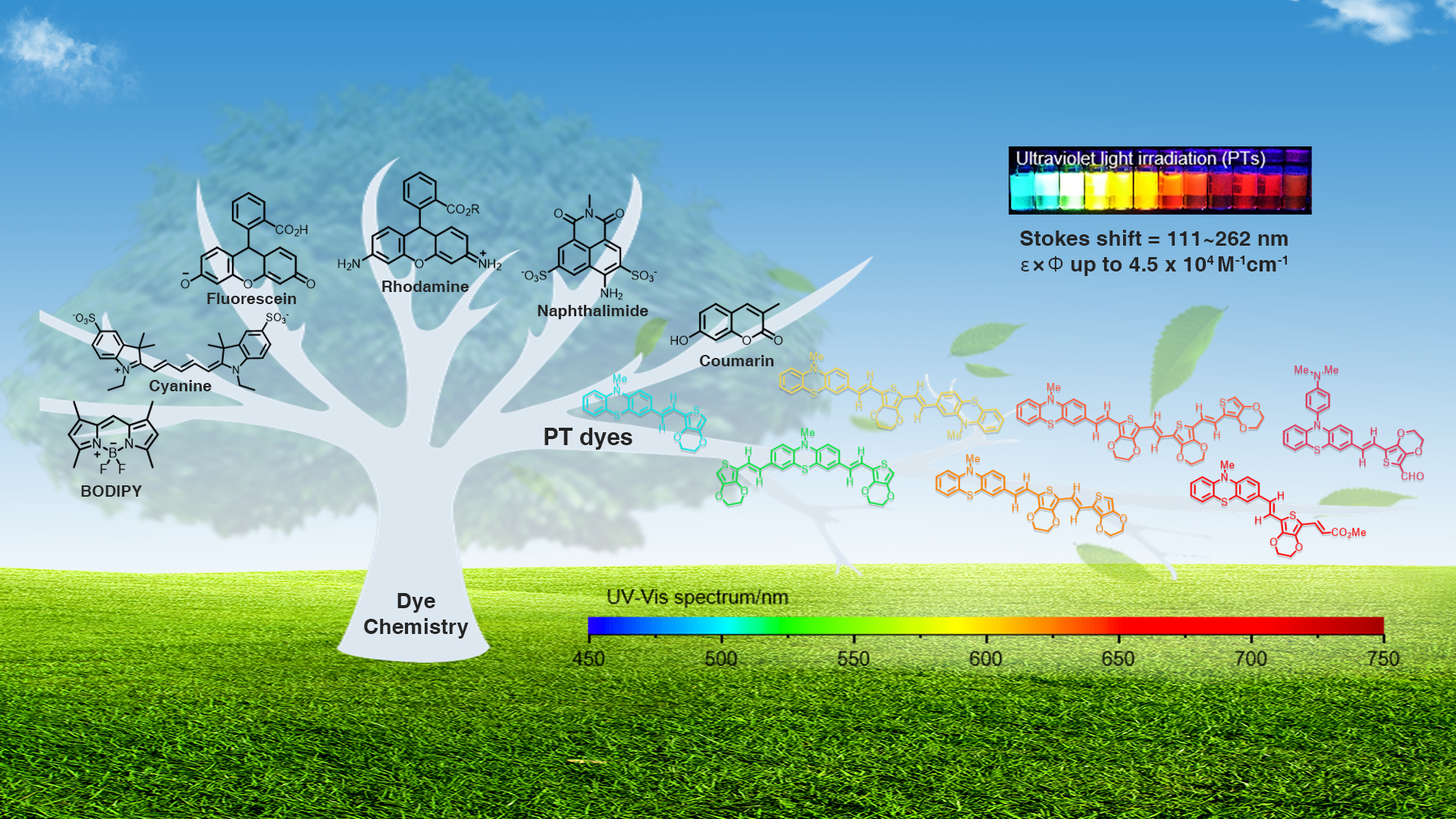
Associate Professor Bo Zhang’s research group from the Department of Biomedical Engineering (BME) at the Southern University of Science and Technology (SUSTech) has recently published a paper that reports a block-building molecular engineering strategy for small-molecule fluorophores and synthesized a series of fluorescent dyes (named PTs) with large Stokes shifts, tunable wavelengths, and balanced brightness, enabling rapid nucleic acids/proteins assays with high sensitivity visible to naked eye.
Their work, entitled “Organic Fluorophores with Large Stokes Shift for the Visualisation of Rapid Protein and Nucleic Acid Assays”, has been published in the renowned international chemistry journal Angewandte Chemie International Edition (Angew. Chem. Int. Ed.).
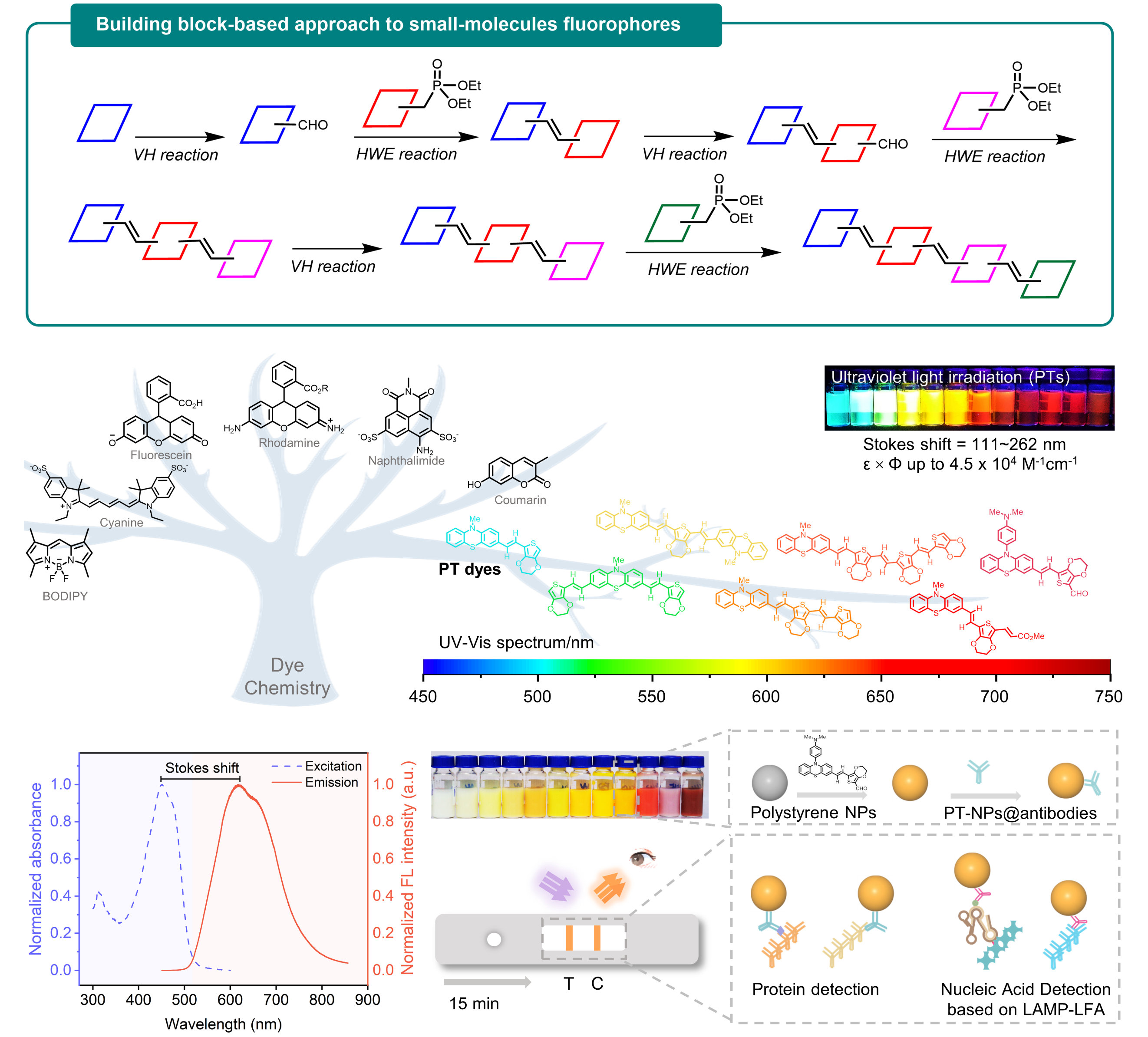
Figure 1. A Block-building molecular engineering strategy for designing small-molecule fluorophores with large Stokes shift and applications in visualization of rapid protein and nucleic acid assays.
Dye chemistry is rapidly evolving from trial-and-error to molecular engineering, emphasizing function-oriented design and synthesis of structures at the molecular level. In this work, a series of small-molecule fluorophores (named PTs) were designed and synthesized via a block-building molecular engineering strategy. They merge phenothiazine moiety with EDOT moiety by π-conjugation, which exhibits long Stokes shift (up to 262 nm), large molar extinction coefficients (ε, 30,000~100,000 M-1cm-1 in DMSO), high quantum yields (Φ, up to 54.8% in DMSO), and flexible wavelength tunability (from visible to near-infrared).
The team also explored the relationship between the electronic energy levels and optical properties of PTs, and according to density-functional theory (DFT), the stacking of molecular blocks and the introduction of electron-absorbing groups can modulate the HOMO-LUMO gap. In addition, the electronic excited states of PTs calculated by Time-dependent density functional theory (TDDFT) are in agreement with the experimental data.
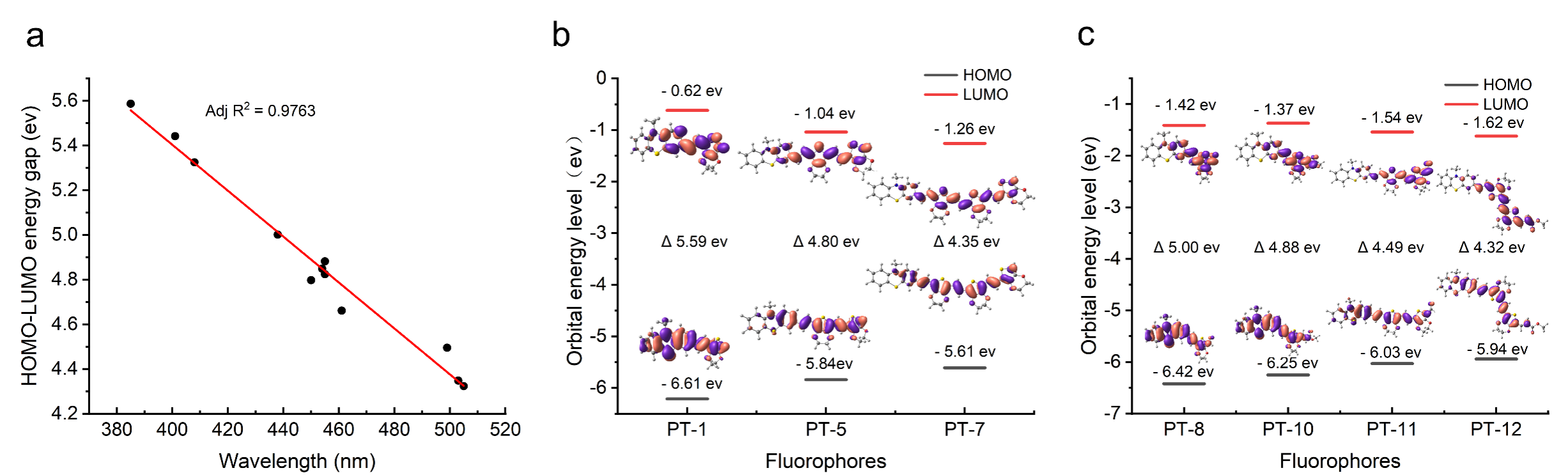
Figure 2. Energy level calculation and electron cloud distribution calculation of PTs by density function theory. (a) Correlation between the LUMO-HOMO energy gap and excitation wavelength of PTs in experiments; (b) LUMO-HOMO energy and electron cloud distribution of PT-1, PT-5, PT-7; (c) LUMO-HOMO energy and electron cloud distribution of PT-8, PT-10, PT-11, PT-12.
PTs dyes were made into fluorescent nanoparticles (PT-NPs), and observed the number of dye molecules encapsulated in a single nanoparticle can reach one million. These bright PT-NPs were applied to lateral flow assay (LFA). Consequently, direct visualization of rapid nucleocapsid (N) protein detection of SARS-CoV-2 with 100-fold sensitivity improvement over colloidal gold-based LFA was achieved, and the limit of detection was 20 fM. This assay was able to pick up more RT-PCR-confirmed SARS-CoV-2 infected clinical samples compared to colloidal gold-based LFA. Besides, the sensitivity of PT-NPs-based LFA also surpasses reported LFAs with various luminescent materials for SARS-CoV-2 detection.
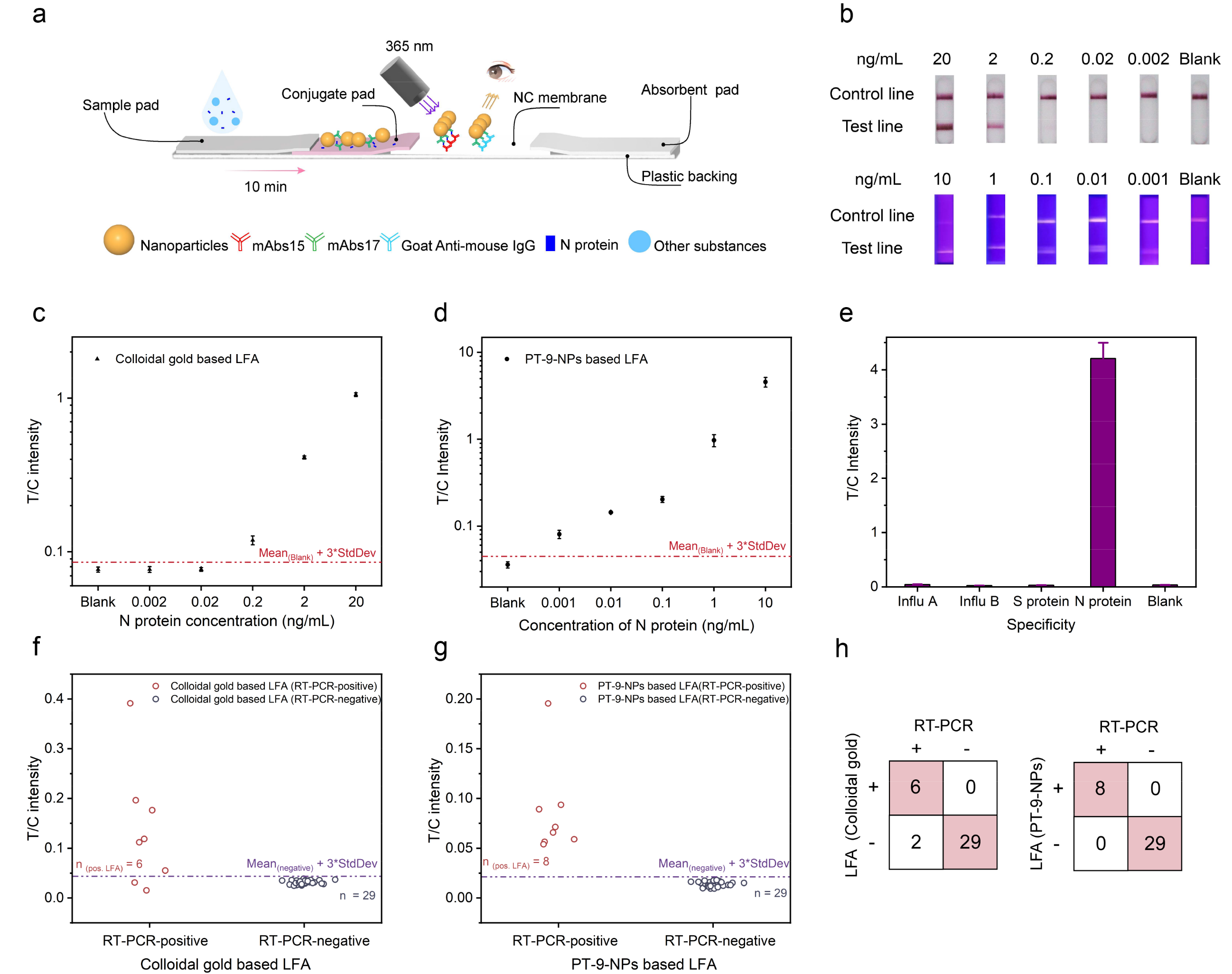
Figure 3. Lateral flow immunochromatography based on PT-NPs for SARS-CoV-2 antigen detection. (a) Schematic diagram of the detection system; (b) Comparison of sensitivity between the present work and colloidal gold assay; (c) Detection curve of the colloidal gold assay; (d) Detection curve of the present work’s assay system; (e) Specificity of the present work’s LFA system; (f-h) Analysis of the clinical assays of the colloidal gold assay and the present work’s LFA.
Since May 2022, the monkeypox epidemic (Mpox) has swept through more than 100 countries and regions, and has been classified as a “Public Health Emergency of International Concern”. In this work, the researchers screened the conserved sequences of monkeypox virus genome by loop-mediated isothermal amplification (LAMP) and designed primer structures adapted to the LFA system based on PT-NPs, which enabled rapid visual nucleic acid detection of monkeypox virus (MPXV) at the single-copy level.
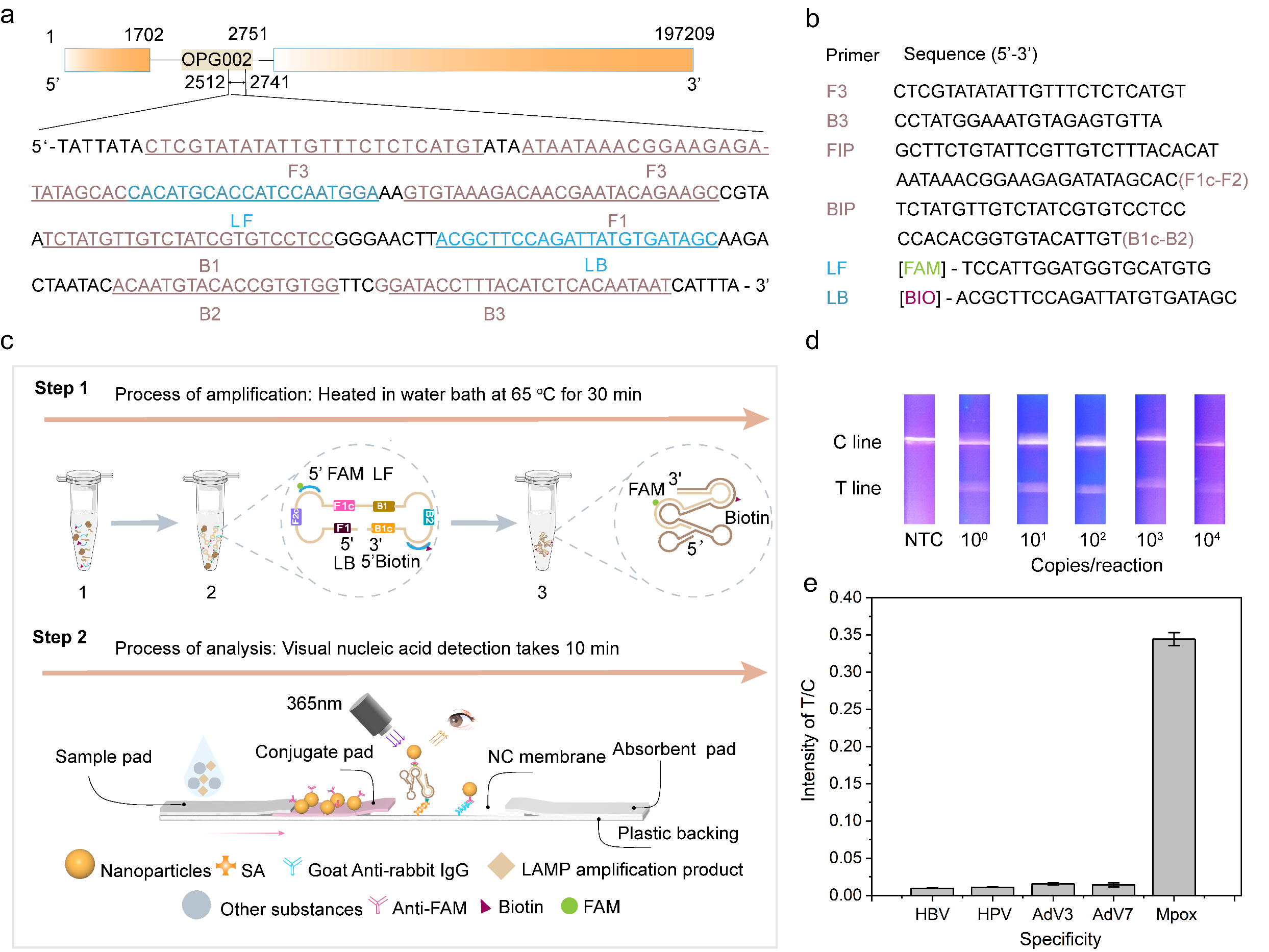
Figure 4. PT-NPs-based lateral flow immunochromatography combined with LAMP technology for monkeypox virus nucleic acid detection; (a) Monkeypox virus nucleic acid detection target sequences; (b) Design of LAMP primer probes for monkeypox virus detection; (c) Schematic diagram of the flow of monkeypox virus nucleic acid detection based on PT-NPs and LAMP; (d) Monkeypox virus detection visualisation of the results of the rapid detection, with a sensitivity of up to single copy; (e) Specificity of the detection system. Sensitivity up to a single copy; (e) Specificity of the detection system.
Since the synthesis of the first unnatural small-molecule fluorescent dye, fluorescein, by humans at the end of the 19th century, dye chemistry has gradually evolved from a trial-and-error process to a disciplined molecular engineering design. This study explores the molecular engineering design of large Stokes shift organic fluorescent dyes, providing an organic conjugated modular design strategy for dye chemistry, as well as new fluorescent materials for in vitro diagnostics to reduce the reliance on expensive optical devices.
Master’s students Jingkai Yang and Ziyi Xu, both from the Department of BME at SUSTech, are the first and co-first authors of this paper, respectively. Associate Professor Bo Zhang is the corresponding author, while Professor Pan-Lin Shao from Guangzhou Medical University (GMU) and Research Assistant Professor Ying Liu from the Department of BME at SUSTech are the co-corresponding authors. SUSTech is the first affiliated unit of the paper.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Fund for Basic and Applied Basic Research, Shenzhen Science and Technology Programme, and Guangdong Key Laboratory of Advanced Biomaterials.
Paper link: https://doi.org/10.1002/anie.202318800