Chris Edwards | 10/03/2020 40
Optical imaging has emerged as one of the promising imaging techniques in both research and clinical practice. Photoacoustic imaging and fluorescent imaging are the two leading representatives of optical-related imaging approaches. Their imaging qualities highly rely on contrast agents. The ideal photoacoustic and fluorescent agent is still to be found. The theoretical system that guides the construction of efficient contrast agents has not been widely established.
Associate Professor Kai Li (Biomedical Engineering – BME) teamed up with several research teams. They have made significant progress in designing near-infrared two-zone imaging materials and biomedical diagnosis. Their research outcomes have been published in Angewandte Chemie International Edition (Angew Chem Int Ed), Biomaterials, and Research.
The teams verified their design concepts and strategies through theoretical calculations. They obtained various high-performance photoacoustic and fluorescent imaging materials with optical activity in the second near-infrared window (NIR-II, 1000-1700 nm). They have applied them in different research fields, such as vascular imaging and tumor detection.
The first paper at Angew Chem Int Ed. was titled “An Ester‐substituted Semiconducting Polymer with Efficient Nonradiative Decay Enhances NIR‐II Photoacoustic Performance for Monitoring of Tumor Growth.”
The research teams synthesized several thiadiazoloquinoxaline (TQ)-based semiconducting polymers (SPs) with a broad absorption covering the NIR-I to NIR-II regions. The excited s-BDT-TQE shows a large dihedral angle, narrow adiabatic energy, and low radiative decay. It is attributed to the strongly electron-deficient ester-substituted TQ-segment.
The more vigorous molecular motions trigger higher reorganization energy. They yield an efficient photo-induced nonradiative decay. BDT-TQE SP-cored nanoparticles with twisted intramolecular charge transfer (TICT) exhibit a high NIR-II photothermal conversion efficiency (61.6%). They also exhibit the preferable PA tracking in-situ hepatic tumor growth for more than 20 days.
The research teams proposed a new approach that adjusted the TICT effect in polymer chains for augmented PNRD property. It enhanced the photothermal conversion & photoacoustic performance for monitoring in-situ tumor growth in mice.
BME masters student Menglei Zha is the co-first author, along with City University of Hong Kong (CityU) Dr. Xiangwei Lin and National Kaoshiung University of Science and Technology (NKUST), Professor Jen-Shyang Ni. Dr. Kai Li is a co-corresponding author of this paper, with CityU Professor Lidai Wang and NKUST Dr. Jen-Shyang Ni.
The authors are grateful to the National Natural Science Foundation of China, Guangdong Innovative and Entrepreneurial Research Team Program, the Shenzhen Science and Technology Program, Research Grants Council of the Hong Kong Special Administrative Region, and Fundamental Research Funds for the Central Universities for financial support. The authors also acknowledge the Center for Computational Science and Engineering at SUSTech for theoretical calculation support and SUSTech Core Research Facilities for their technical support.
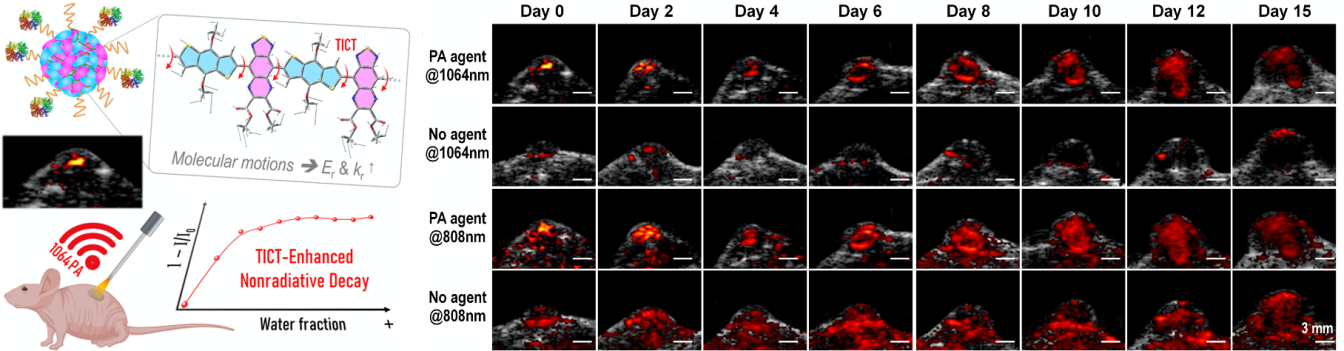
Figure 1. Improve the nonradiative transition efficiency of semiconductor polymers to achieve long-term real-time monitoring of tumor growth via NIR-II photoacoustic imaging
The paper published in Biomaterials was titled, “Self-assembled AIEgen nanoparticles for multi-scale NIR-II vascular imaging.” It looked at how fluorogens with aggregation-induced emission (AIEgens) have been intensively explored in biomedical applications.
One primary strategy to bring these hydrophobic AIEgens into the aqueous biological environment is to encapsulate them in nanoparticles with functionalized polymeric matrices. Most fluorophores are insoluble in water, requiring post-modification like amphiphilic polymer encapsulation. The traditional preparation of AIEgen nanoparticles is convenient. These methods are hard to fulfill the required uniform size and stable loading efficiency. The exploration of reliable strategies that can afford AIE nanoparticles with the above requirements with minimized batch-to-batch variation remains challenging.
The research team designed amphiphilic AIEgens, built with a hydrophobic donor-acceptor-donor (D-A-D) core and hydrophilic polyethylene glycol (PEG) chain. These AIEgens can self-assemble into uniform nanoparticles with average sizes of ~35 nm, showing an emission maximum beyond 1000 nm and quantum yields (QYs) above 10%. The team used the bright AIE nanoparticles for multi-scale intravital vascular fluorescence imaging in the second near-infrared window (NIR-II, 1,000-1,700 nm) in mouse and rabbit models.
Advanced AIE nanoparticles with efficient self-assembly strategies were constructed for angiography. The early stages of demonstration for the AIEgen nanoprobes for NIR-II imaging in large animal models (rabbits with an average weight of 3 kg) will inspire more research opportunities in designing advanced NIR-II probes and promoting the translational studies of this emerging imaging technique.
BME doctoral student Yaxi Li and Shenzhen Institute of Advanced Technology (SIAT) Dr. Dehong Hu are the co-first authors. Dr. Kai Li and SIAT Dr. Hairong Zhang are the co-corresponding authors of this paper.
This work was supported by the National Natural Science Foundation of China, Guangdong Innovative and Entrepreneurial Research Team Program, the Science and Technology Innovation Committee of Shenzhen Municipality, the Shenzhen key laboratory of ultrasound imaging and therapy, and CAS key laboratory of health informatics. The authors also acknowledge the Center for Computational Science and Engineering at SUSTech for theoretical calculation support and SUSTech Core Research Facilities for technical support.
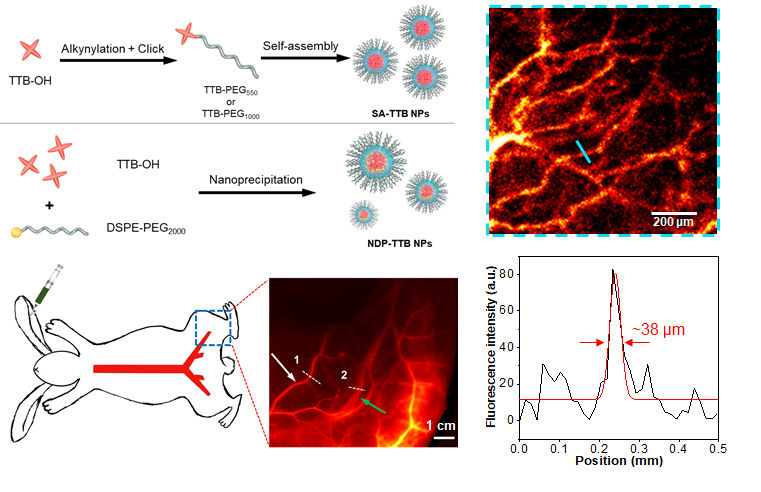
Figure 2. Self-assembly strategy to prepare aggregation-induced luminescence nanoprobes to achieve multi-scale NIR-II vascular imaging
The paper published in Research was titled, “Centimeter-Deep NIR-II Fluorescence Imaging with Nontoxic AIE Probes in Non-Human Primates.” In biomedical fluorescence imaging, AIEgens plays an increasingly important role. However, there is still a lack of in-depth toxicological research and deep imaging evaluation in primate models.
The research team studied the high intravenous injection dose of AIE probes, 30 times the standard clinical dosage in primate blood and histological analysis report. The results showed that the AIE probes were in the normal range after 35 days of metabolism in primates, which proved that the AIE probes were not biologically toxic. The research team also utilized the biological safety and high-brightness optical performance of the AIE probe. They successfully achieved a depth of 1.5cm blood vessel imaging in primates, breaking the current limit of near-infrared two-zone fluorescence imaging at the millimeter level.
The two features of NIR-II AIE probes enable them to become promising fluorophore candidates for angiography and lymphadenopathy. They could pave the way to promote clinical translation of NIR-II AIE probes in human clinical trials.
Dr. Kai Li is a co-corresponding author of this paper, with the Hong Kong University of Science and Technology (HKUST) Professor Ben Zhong Tang and SIAT Dr. Hairong Zheng. Additional contributions came from the National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital & Shenzhen Hospital.
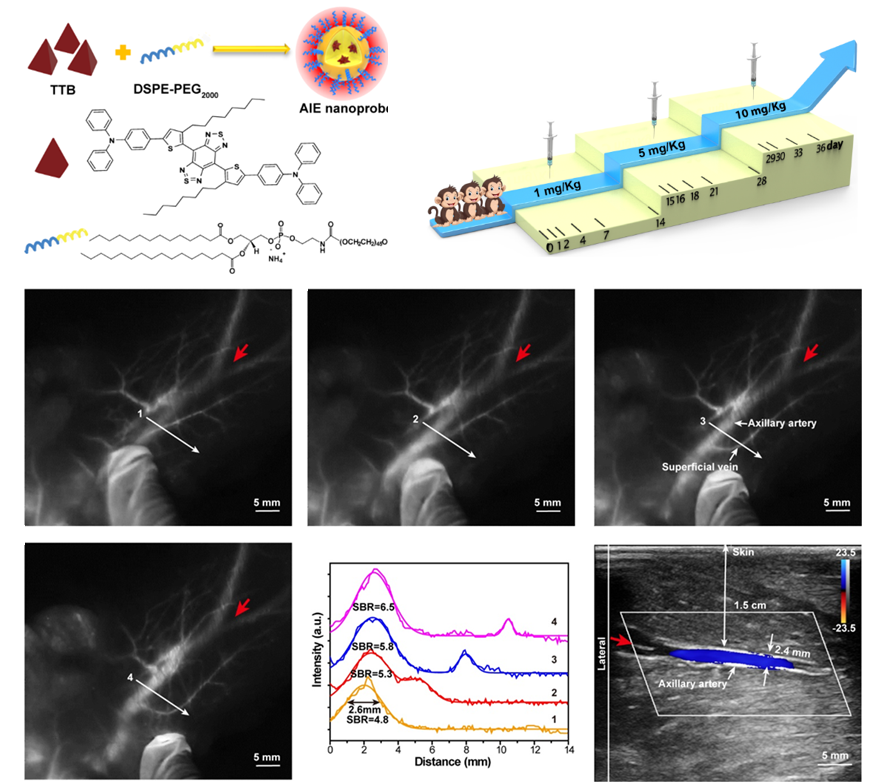
Figure 3. The NIR-II AIE probe achieves high signal-to-noise ratio blood vessel imaging in primates with a depth of 1.5 cm
The second paper published in Angew Chem Int Ed. was titled “Sub‐10 nm Aggregation‐Induced Emission Quantum Dots Assembled by Microfluidics for Enhanced Tumor‐Targeting and Reduced Retention in the Liver.” AIE dots (AIE dots) obtained by hybridization with phospholipids have been widely used in biomedical imaging. However, the particle size of the AIE dots prepared in this traditional method is usually larger than 25 nm. Due to the interception of the liver, spleen, and other organs, the ideal imaging effect is often difficult to obtain with such large AIE dots.
The research team developed a microfluidic chip with a double spiral mixing pipe (pipe width 300 microns, height 60 microns) to prepare AIE nanoparticles. These are known as AIE quantum dots (QDs), separate from the larger AIE dots. AIE QDs allow more efficient cellular uptake and imaging without surface modification of any membrane-penetrating peptides or other targeting molecules.
NIR-II AIEgens were used to demonstrate that AIE QDs can achieve high contrast at the tumor as small as 80 mm3 and evade the liver more efficiently than AIE dots.
The sub-10 nm organic AIE QDs were successfully synthesized in a microfluidic chip, showing better in-vitro and in-vivo solid-tumor imaging than AIE dots. These AIE QDs promises a pathway for efficient cell labeling and sensitive & precise detection of the tumor. The microfluidic method may help make a broad range of multi-function AIE molecules into sub-10 nm AIE QDs. They could have immense potential in diverse biological applications.
Doctor Xuanyu Li from the University of the Chinese Academy of Sciences (UCAS) is the first author, and Menglei Zha is the co-first author. Dr. Kai Li is a co-corresponding author. Additional contributions came from the BME Microfluidic Research laboratory.
The research teams thank the National Key R&D Program of China, the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Guangdong Innovative and Entrepreneurial Research Team Program, the Science and Technology Innovation Committee of Shenzhen Municipality, and the Tencent Foundation through the XPLORER PRIZE for financial support. The authors also acknowledge the Center for Computational Science and Engineering at SUSTech for theoretical calculation support and SUSTech Core Research Facilities for technical support.
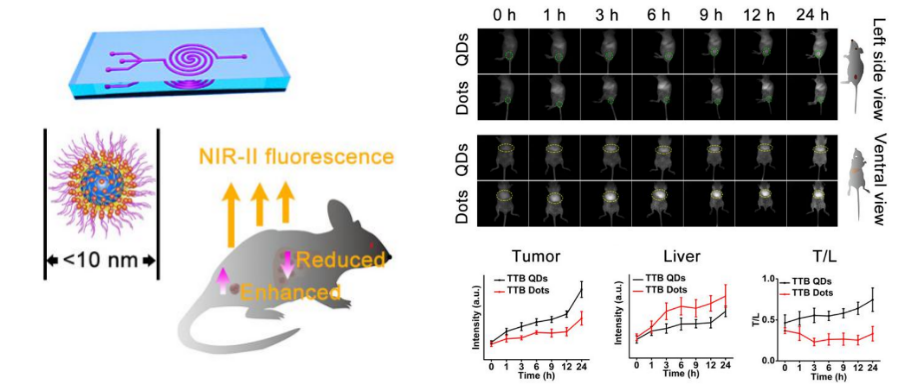
Figure 4. The new microfluidic synthesis strategy prepares sub-10 nm AIE QDs to reduce liver retention and enhance tumor targeting in tumor-bearing mice.
Related links:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202010228
https://www.sciencedirect.com/science/article/pii/S0142961220306116
https://doi.org/10.34133/2020/4074593
https://onlinelibrary.wiley.com/doi/10.1002/anie.202008564