Research | 2020-05-21
BackNew strategies have been developed at Southern University of Science and Technology (SUSTech) that could see cancer treated in a less invasive manner.
Photothermal therapy (PTT) using near-infrared (NIR) light-absorbing agents to generate heat for tumor ablation locally has received considerable interest in recent years. PTT has become an important research direction for cancer treatment. However, traditional PTT methods suffer from several limitations, including complex synthesis of inorganic/organic photothermal agents (PTA), using high laser power density, and tissue damage from the high-temperature PTT.
The latest progress in applying low-temperature photothermal therapy examined the synthesis of small molecule PTAs with high PTCE, as there is enormous potential for biomedical applications.
Associate Professor Kai Li (Biomedical Engineering) has led his research group to publish a ground-breaking paper in the high-impact academic journal, Angewandte Chemi International Edition (Angew Chem Int Ed) (IF = 12.257). The paper was titled, “Photoinduced Nonadiabatic Decay-guided Molecular Motor Triggers Effective Photothermal Conversion for Hyperthermia Cancer Therapy.”
Their paper has made significant progress in studying the synthetic method of small molecule photothermal agents, and their applications in low-temperature photothermal therapy (PTT). PTT is an important research direction for cancer treatment. However, there are several side-effects and problems with traditional PTT techniques. It means that there is a significant need to develop a new photothermal agent-mediated low-temperature PTT strategy.
Their paper designed a new type of organic small molecules that are based on light-induced, non-adiabatic decay (PIND) effect. The co-delivery of the photothermal molecule with a heat shock protein 70 (HSP70) inhibitor (Apo) leads to suppressed HSP70 expression and realize a high-efficiency PTT tumor treatment at 43°C.
Associate Professor Jen-Shyang Ni, a fellow researcher, explained that when this sort of imine-based molecular motor is irradiated by lasers to an excited state, it will be affected by the strong intramolecular twisted charge transfer effect (TICT). The TICT supports passing through the conical cross (CI) process, which releases energy back to the ground state. It can be considered as a photo-induced non-adiabatic decay (PIND) phenomenon, which has almost no fluorescence emission. They can better convert light to heat and exhibits up to 90% efficiency, compared to existing commercial products.
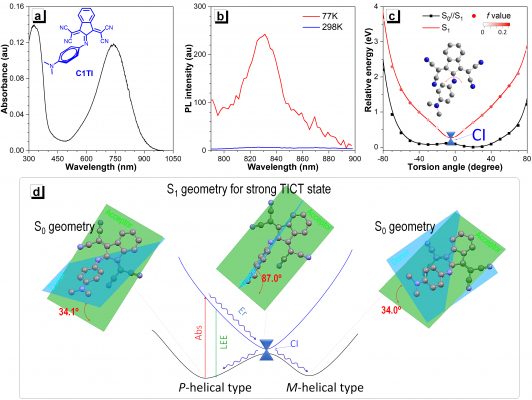
Figure 1. The photophysical properties and working principle of light-induced non-adiabatic decay (PIND) organic small molecules
In animal experiments, the researchers developed a delivery system for tumor cells that used the thermal response technique. Following further experimental processes, they showed that their technique had a significantly better treatment effect than the control group. It proved the effectiveness of a combined treatment strategy, showing an efficient and straightforward photothermal conversion molecular motor that negates the need for introducing long-branch organic alkyl chains or other bulky substituents. Effectively breaking through the traditional limitations has opened many doors for new ideas in the development of small molecule, high-efficiency, photothermal agents.
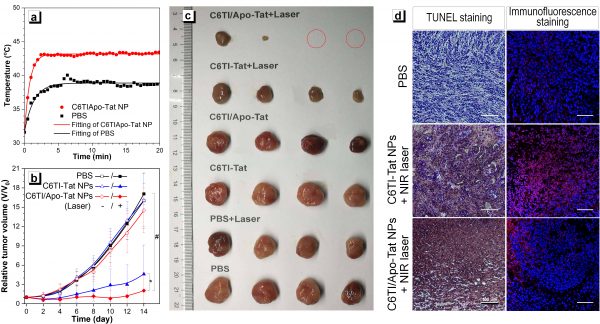
Figure 2. C6TI/Apo-Tat NPs-mediated hypothermic PTT tumor therapy. (a) Temperature curve of 808 nm laser (0.5 W cm-2) irradiated mice tumor site with time; (b) Tumor growth curve of tumor size with the time of different treatment groups; (c) Day 14 of different treatment groups Dissected tumor photographs; (d) HSP70 immunofluorescence staining and TUNEL staining analysis of in situ tumor tissue sections, scale = 100 μm
SUSTech is the first communication unit of the thesis. Associate Professor Jen-Shyang Ni is a co-first author of the paper. Associate Professor Kai Li was the sole correspondent author of the paper. Other significant contributions came from the HKUST-Shenzhen Research Institute and the City University of Hong Kong Shenzhen Research Institute.
The authors received support from the National Natural Science Foundation of China, the Science and Technology Plan of Shenzhen, and the High-Level Special Funds of SUSTech. They also acknowledge the Center for Computational Science and Engineering at SUSTech for theoretical calculation support, and the SUSTech Core Research Facilities for technical support. All in vivo procedures were approved by the Animal Ethics Committee of the Laboratory Animal Research Center of SUSTech.
Paper link: https://www.onlinelibrary.wiley.com/doi/10.1002/anie.202002516
Group introduction
Associate Professor Kai Li: http://faculty.sustech.edu.cn/lik/
Research Associate Professor Jen-Shyang Ni: http://faculty.sustech.edu.cn/nizx/