Research | 2021-12-22
BackYekkuni L. BALACHANDRAN | 22/12/2021
Controlling the spread of the human immunodeficiency (HIV) virus might be the only feasible route for completely avoiding the intractable disease. However, crucial immunities are still difficult to be sufficiently triggered by vaccines alone, which greatly limits its practical application.
Researchers from the Microfluidic-Biomaterials Lab at the Southern University of Science and Technology (SUSTech) have developed a two-dimensional nano-adjuvant comprising of rare-earth elements that improve the immunities generated by vaccines (HIV DNA vaccine in this case).
The research article, titled “Two-dimensional nanosheets as immunoregulator improve HIV vaccine efficacy,” was published in Chemical Sciences, a scientific journal covering all aspects of chemistry.
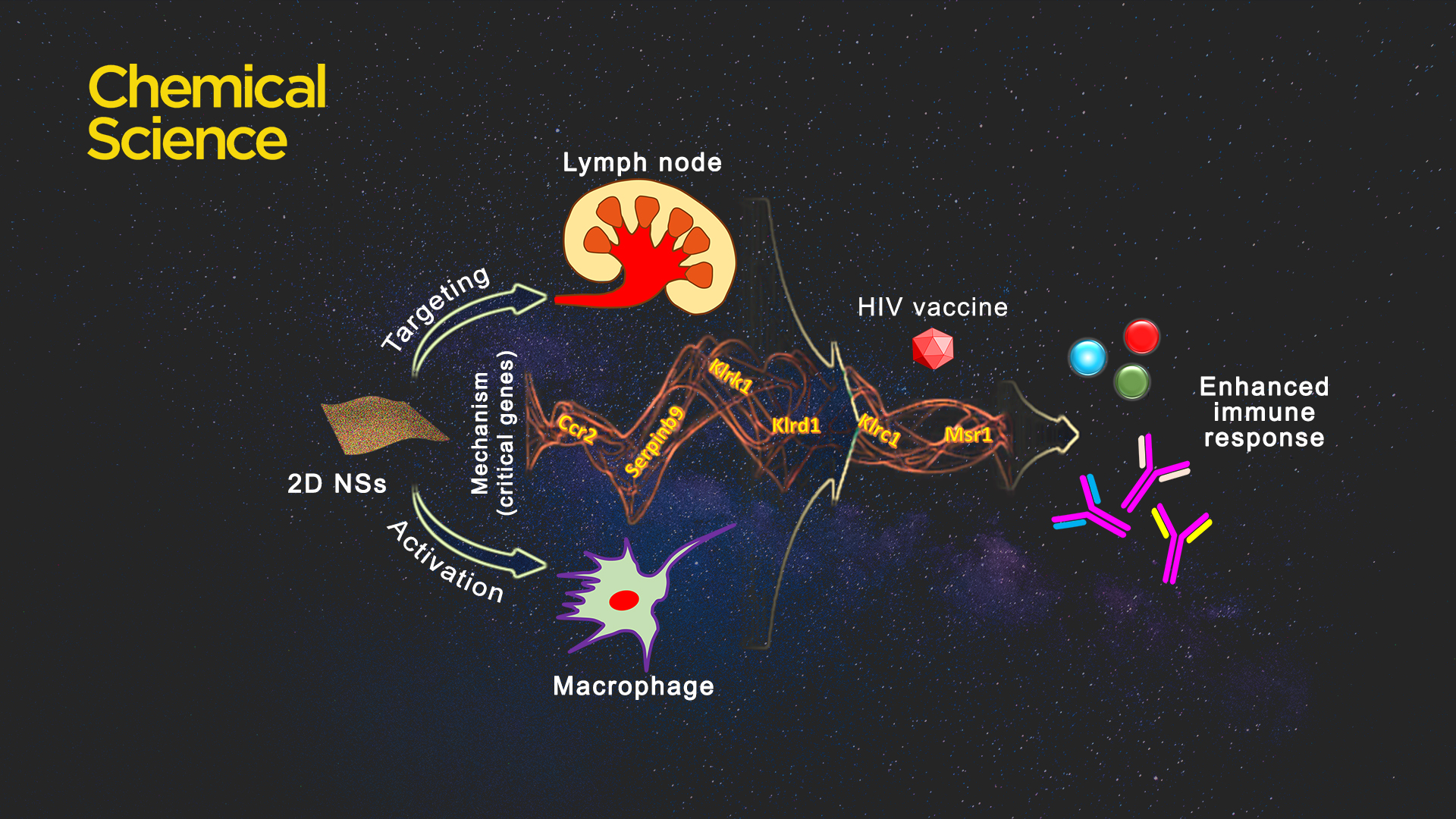
Introducing applicable immunoregulatory building blocks to endow planar materials with inherent immunoregulatory characteristics is a strenuous task. Some rare earths or rare-earth based complexes, because of their inherent immunoregulatory capability, can regulate the behavior of macrophages (antigen-representing cells) and immunity. Considering that multiple types of rare earths have immunoregulatory effects on the functions or behaviors of immune effector cells, selecting rare earths rationally is a core question for the design of immunoregulatory nanomaterials.
Two rare earths, erbium (Er) and dysprosium (Dy), can activate macrophages and improve phagocytosis capability and bioactivity for the increased presentation of antigens. The heterogeneity of two-dimensional nanosheets (2D NSs) comprehensively regulates immune functions of both Er (ROS-based mechanism) and Dy (NO-based mechanism), in comparison with either Er- or Dy-alone 2D NSs.
Because of their planar morphology, these 2D NSs target the mice lymph nodes without the use of any lymph node targeting functional molecules. Considering lymph nodes are a critical type of immunological tissue for mediating immune responses, the lymph node targeting capability of nanosheets effectively improve the efficacy of vaccines.
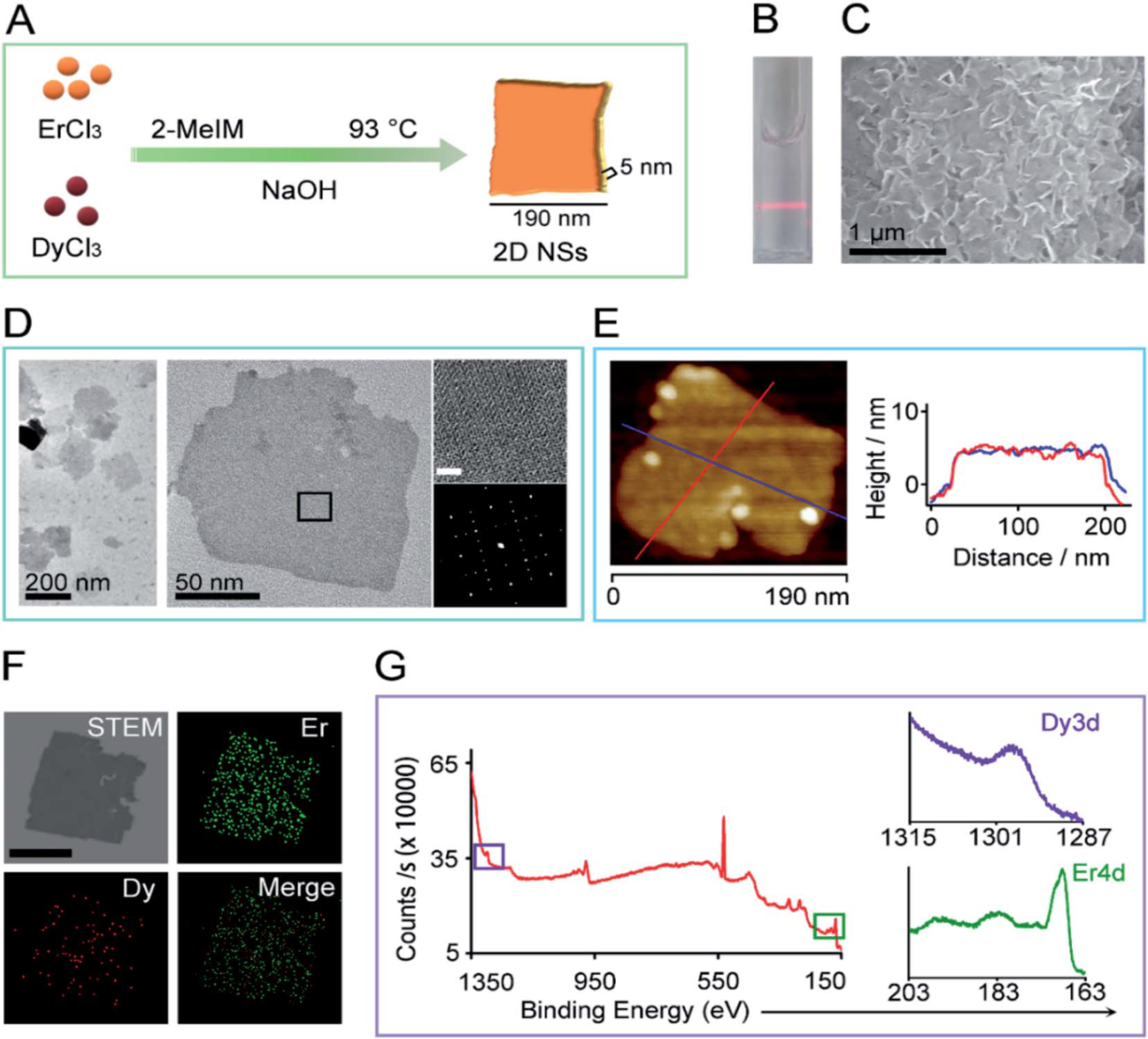
Figure 1. Characterization of rare-earth 2D NSs.
In regulating DNA vaccine-triggered immune responses, the 2D NSs simultaneously improve both humoral and cellular immune responses, compared to most other reported immunoregulators, which can only enhance either humoral or cellular responses. 2D NSs significantly improve the HIV-specific humoral response of IgG and the four subclasses (IgG1, IgG2a, IgG2b, and IgG3).
Furthermore, the 2D NSs can boost the enhancement of cytotoxic T lymphocytes to produce HIV-specific IFN-γ to neutralize HIV-infected cells. The balanced enhancement of HIV-specific humoral and cellular immune responses regulated by 2D NSs gives an unparalleled advantage for realizing the neutralization (mediated by humoral response) and cytotoxicity (cellular response) against HIV.
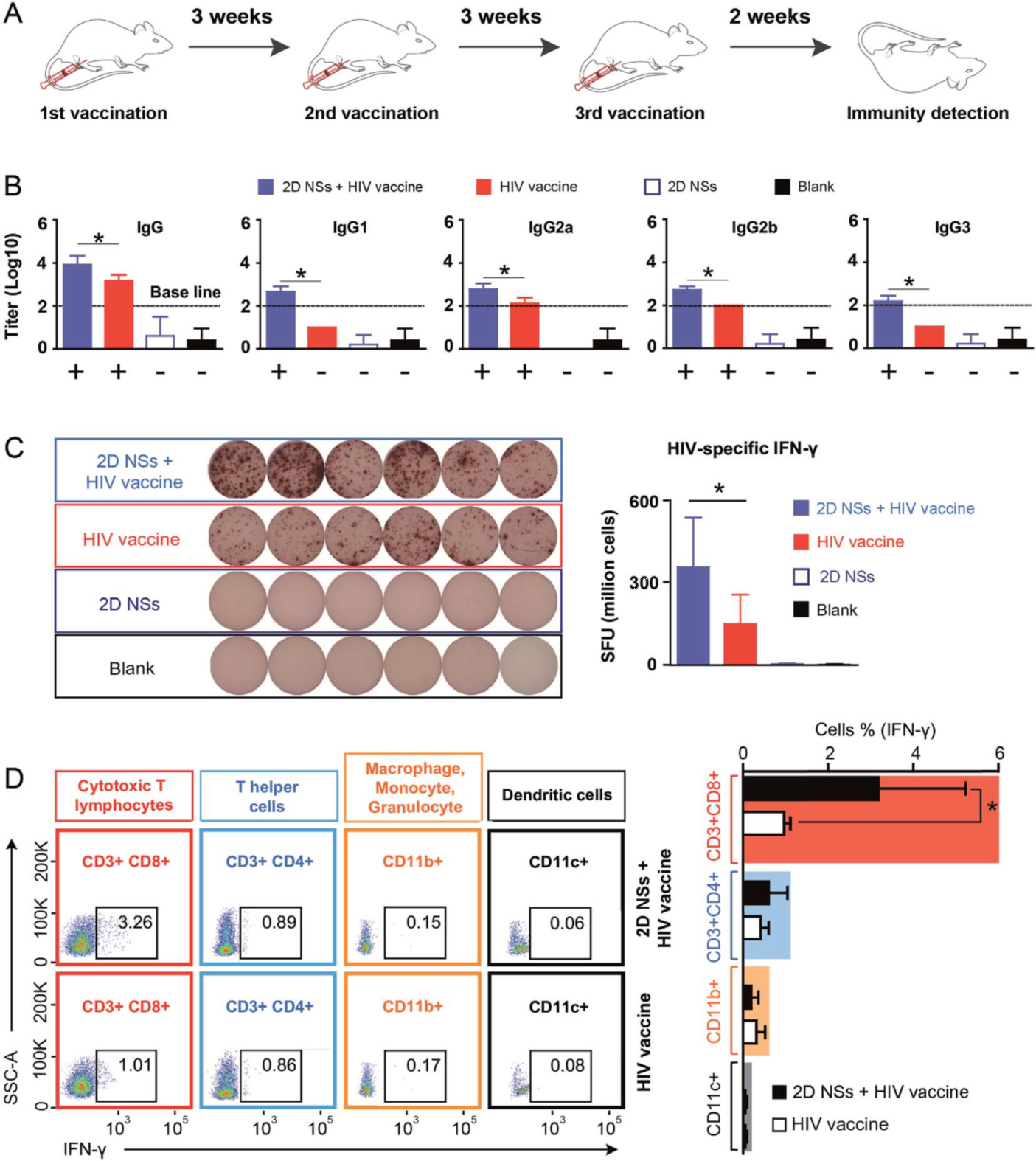
Figure 2. 2D NSs regulating HIV DNA vaccine-triggered immune responses
2D NSs-regulated HIV vaccine triggers six critical genes associated with various immunoregulation-related networks. The three natural killer cell lectin-like receptor subfamily genes Klrk1, Klrd1, and Klrc1, are triggered by nanosheets, which can effectively enhance the presentation of antigens. The 2D NSs induce the upregulation of the genes Ccr2 and Serpinb9 to regulate cytokine production. The 2D NSs are found to significantly up-regulate the expression of Msr1, a critical gene that activates the macrophages. The 2D NSs influences the immunoregulation-related network involving the activation of immune cells, antigen presentation, and the production of immune effectors to facilitate the HIV DNA vaccine to trigger stronger immune responses.
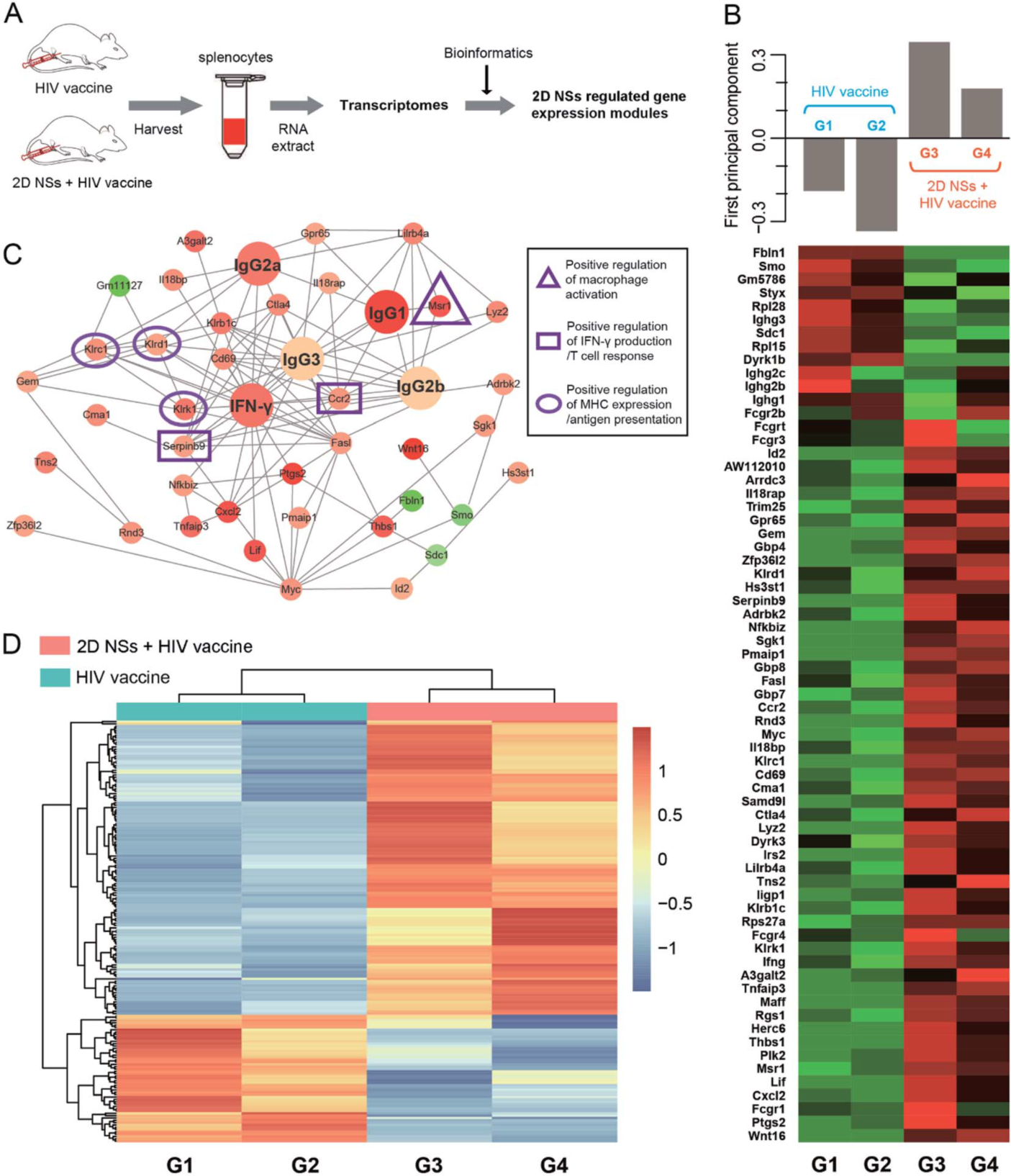
Figure 3. Transcriptome profiling of 2D NSs improving HIV DNA vaccine
In summary, the realization of the concept of 2D NSs immunoregulator dramatically broadens the choices to optimize the vaccination of infectious diseases, tumor immunotherapy, and other immune-based preventive treatment and therapy.
The Microfluidic-Biomaterials Lab at SUSTech is the corresponding author of this paper.
This work was supported by the National Natural Science Foundation of China (NSFC), the National Key R&D Program of China, the Chinese Academy of Sciences, Tencent Foundation through the XPLORER PRIZE, the Shenzhen Key Laboratory of Smart Healthcare Engineering, the Leading Medical Talents Program of Health Commission of Yunnan Province, the Young and Middle-Aged Academic and Technical Leaders Program of Yunnan Province, and the Basic Research Program of Yunnan Province.
Paper link: https://pubs.rsc.org/en/content/articlelanding/2022/SC/D1SC04044H