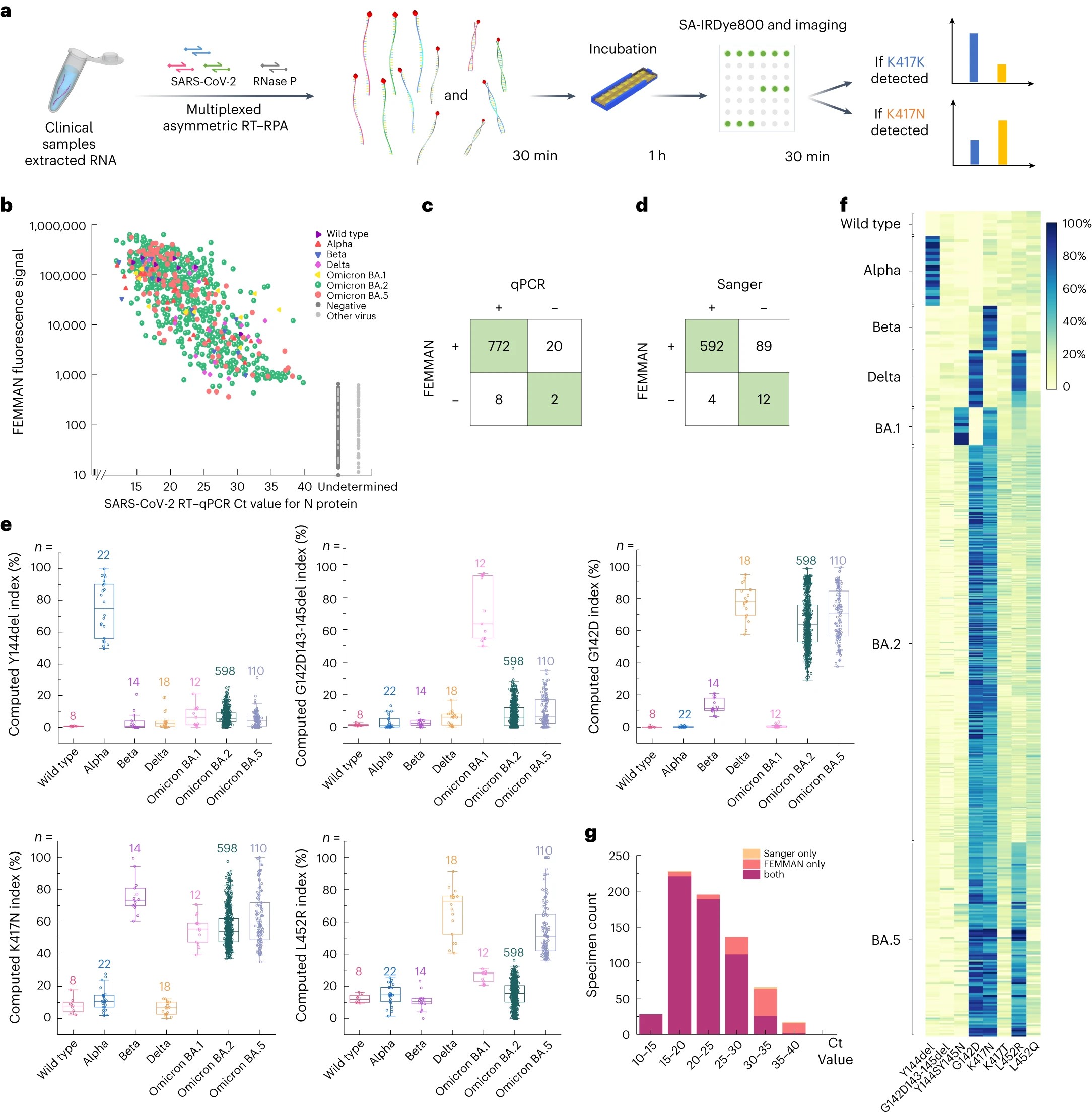
As biotechnology advances, large-molecule drugs such as peptides, proteins, and nucleic acids are increasingly gaining widespread clinical applications. These macromolecular agents display superior targeting and therapeutic efficacy compared to small-molecule drugs, and are applicable to a variety of diseases that threaten human health, including cardiovascular diseases, diabetes, and cancer. However, one significant obstacle is their susceptibility to degradation in the gastrointestinal tract and their large molecular size, which hampers effective oral absorption.
Despite considerable research investment in developing new delivery technologies over the past decades, very few orally administered peptide-based drugs have received FDA approval. For instance, the oral formulation of semaglutide, Rybelsus, approved by the FDA in 2020, still has a bioavailability of less than 1%, even after the addition of a multitude of absorption-promoting agents. To address this challenge, recent research efforts have sought to develop devices that can directly inject the drug into the gastrointestinal mucosa. However, the stability and safety of these technologies remain under scrutiny.
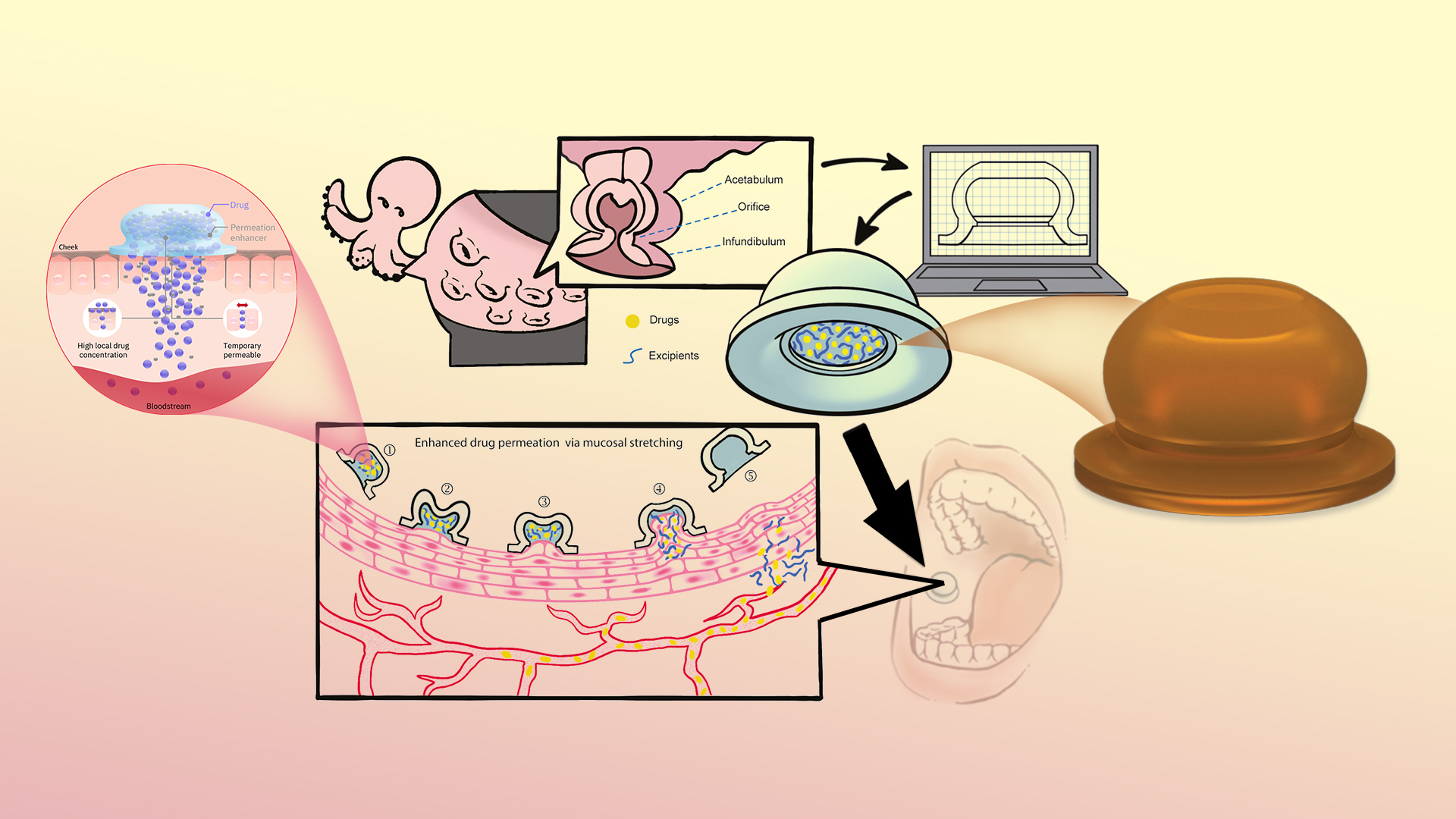
A team led by Associate Professor Zhi Luo of the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) has recently published a study inspired by the unique structure and efficient absorptive properties of octopus suckers, that leverages 3D printing technology to design a non-invasive oral drug delivery device.
Their research paper, entitled “Boosting systemic absorption of peptides with a bioinspired buccal-stretching patch”, has been published in the journal Science Translational Medicine.
The technology created by the researchers mimics the three-dimensional structure and muscle arrangement of octopus suckers to achieve gentle but effective adhesion and stretching on the oral mucosa. This mechanical force can reversibly disrupt the epithelial cell structure and destroy the lipid barrier, thereby facilitating the efficient diffusion of peptide molecules. Furthermore, this delivery device can be loaded with various types of excipients, including absorption promoters and release modifiers, to achieve controlled release of peptide drugs. Thus, through the synergistic action of mechanical stretching and chemical absorption enhancers, this formulation method efficiently diffuses the drug into the highly vascularized mucosal tissue, promoting its entry into the systemic circulation.
This work also marked improvement in bioavailability: In in vivo studies using a large animal model (beagle dogs), the bioinspired suction patch demonstrated two orders of magnitude increase in the bioavailability of desmopressin, a peptide drug whose oral bioavailability is only 0.1%, compared to commercial tablet formulations. For semaglutide, this formulation showed bioavailability comparable to the FDA-approved Rybelsus tablets.
Representing a novel oral peptide drug formulation, a PCT patent application has been submitted for this work. The formulation is a non-invasive drug delivery technology with a simple, highly flexible process suitable for the delivery of various large-molecule drugs. Human trials involving 40 healthy participants verified the high acceptability of the new formulation, indicating substantial clinical translational potential.
The study offers a novel and practical approach to resolving the challenges of oral absorption of large-molecule drugs. The synergistic effect of mechanical stretching and chemical absorption enhancers introduces a novel mechanism for the oral delivery of macromolecular drugs. This delivery modality has the potential to become a platform technology for various types of macromolecular drug formulations, providing more convenient and effective treatment options for patients.
Assoc. Prof. Zhi Luo is the first and corresponding author of this paper, and SUSTech is the first affiliation unit. Prof. Jean-Christophe Leroux at the Swiss Federal Institute of Technology in Zurich is a co-corresponding author.
This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology and the Guangdong Provincial Key Laboratory of Biomaterials, among other projects.
Paper link: https://www.science.org/doi/10.1126/scitranslmed.abq1887