Author: admin
Researchers make advances in field of transarterial chemoembolization modeling for liver cancer treatment
Hepatocellular carcinoma (HCC) accounts for more than 90% of primary liver cancer and remains a worldwide health problem due to its aggressive and lethal nature. Transarterial chemoembolization (TACE) is the first-line treatment for unresectable HCC. This treatment involves the embolization of tumor vessels and the delivery of high-concentration chemotherapy drugs, which are locally released into the tumor tissues to promote tumor necrosis. However, the therapeutic effect of this method has always been controversial. Therefore, designing an ideal embolization model to evaluate the pharmacokinetics inside the tumor site and accurately evaluate the therapeutic effects of clinically relevant embolization agents is of great significance.
Assistant Professor Qiongyu Guo’s team from the Department of Biomedical Engineering and Assistant Professor Xiaoying Tang’s team from the Department of Electronic and Electrical Engineering at the Southern University of Science and Technology (SUSTech) have recently collaborated on the publication of a visualized 3D tumor-mimicking chemoembolization model. The model, which combines deep learning and graph analysis methods, has made significant progress in the construction of an ex vivo organ-structured evaluation platform for liver cancer chemoembolization treatment.
Their research results, entitled “A 3D Tumor-Mimicking in Vitro Drug Release Model of Locoregional Chemoembolization Using Deep Learning Based Quantitative Analyses,” have been published in Advanced Science.
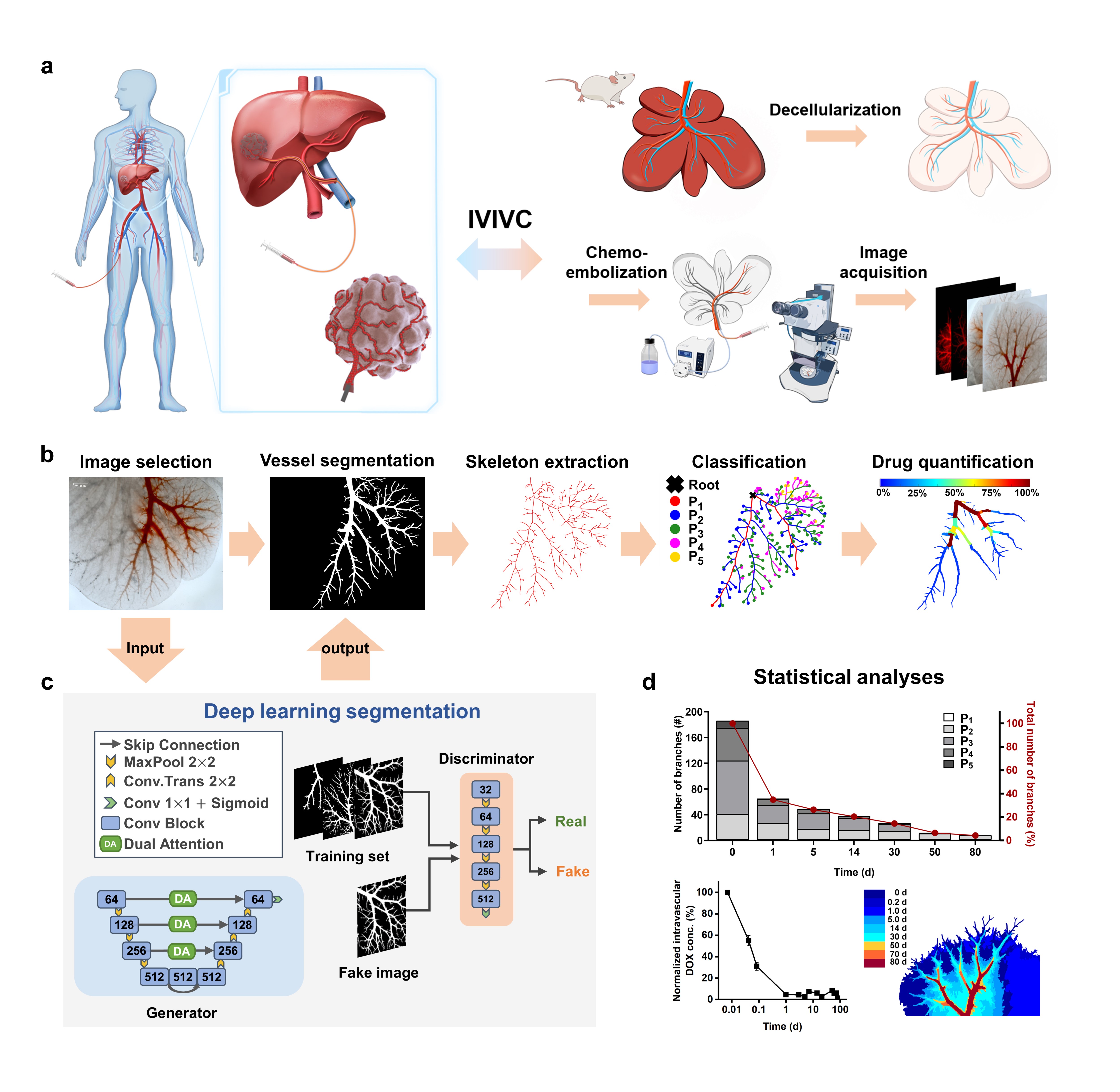
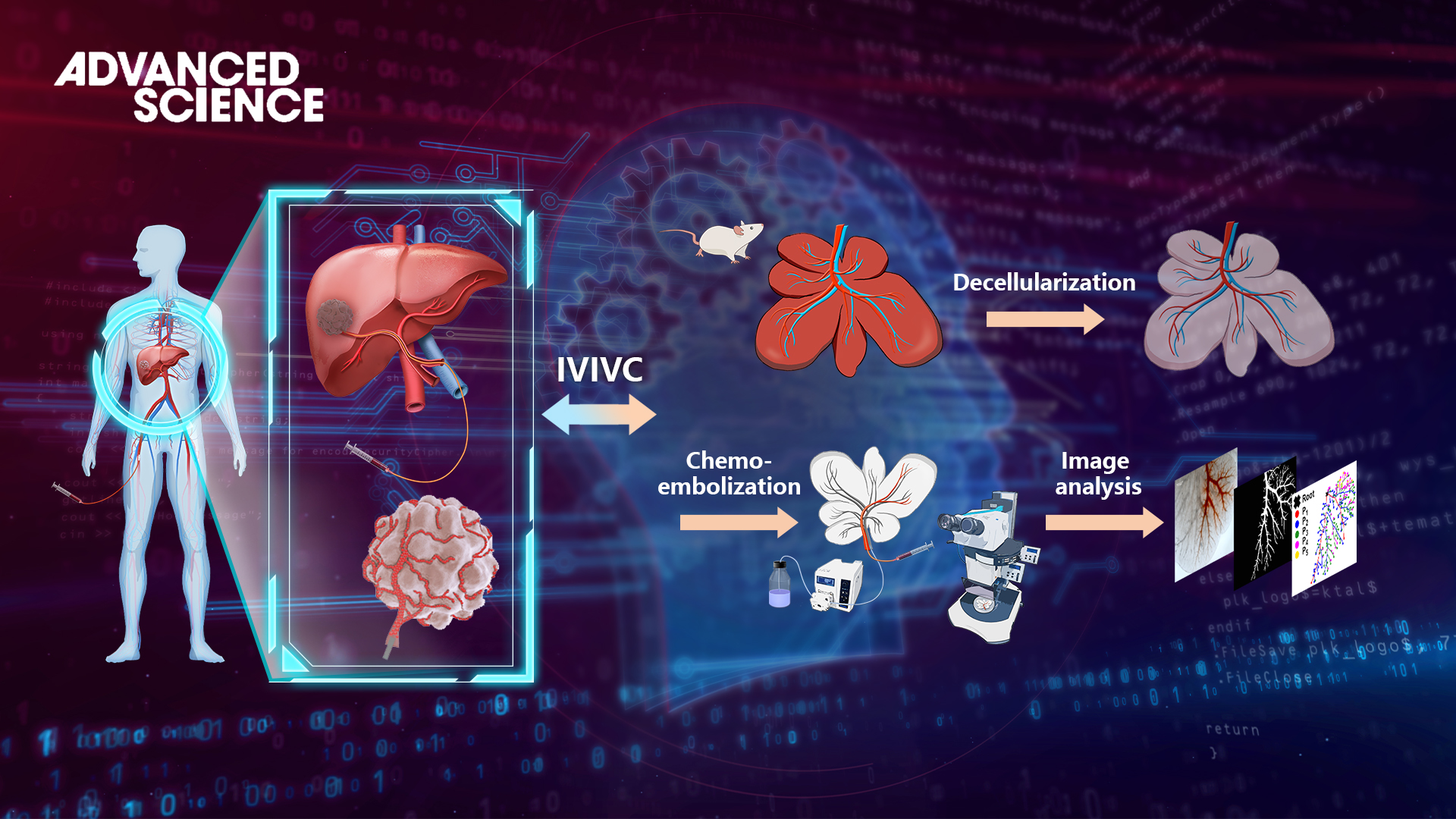
Figure 1. Schematic diagram of the 3D embolization model based on deep learning.
Prof. Qiongyu Guo’s and Prof. Xiaoying Tang’s teams collaborated to design a 3D tumor-mimicking drug release model through utilizing decellularized liver organ as a drug-testing platform (Figure 1). The model contains three key features that affect drug release in vivo: a complex vascular system, a drug-diffusible electronegative extracellular matrix, and controllable drug depletion. This drug release model, combined with deep learning-based computational analyses for the first time, permits quantitative evaluation of all important parameters associated with locoregional drug release. The U-shaped segmentation network based on attention mechanism and adversarial training can achieve accurate segmentation of the embolized vascular region with few annotated samples.
A series of image processing and graph analysis algorithms were combined to achieve an accurate and automatic quantitative statistical analysis of drug loss. To further verify the feasibility and accuracy of the model, the study quantitatively analyzed the endovascular embolization distribution, intravascular drug retention, and extravascular drug diffusion, establishing an in vivo-in vitro correlation with in-human results up to 80d.
Using the established 3D tumor-mimicking model, the in vitro chemoembolization efficacy of three different formulations of doxorubicin solution was systematically evaluated (Figure 2). The researchers used image processing algorithms to extract the skeleton of the segmented embolized vessels and constructed a custom multi-level tree. Subsequently, quantitative analysis and statistical comparison were performed at different tree levels, allowing for a clear depiction of the differences in embolization depth and changes in residual drugs within blood vessels for the three formulations over 80 days.
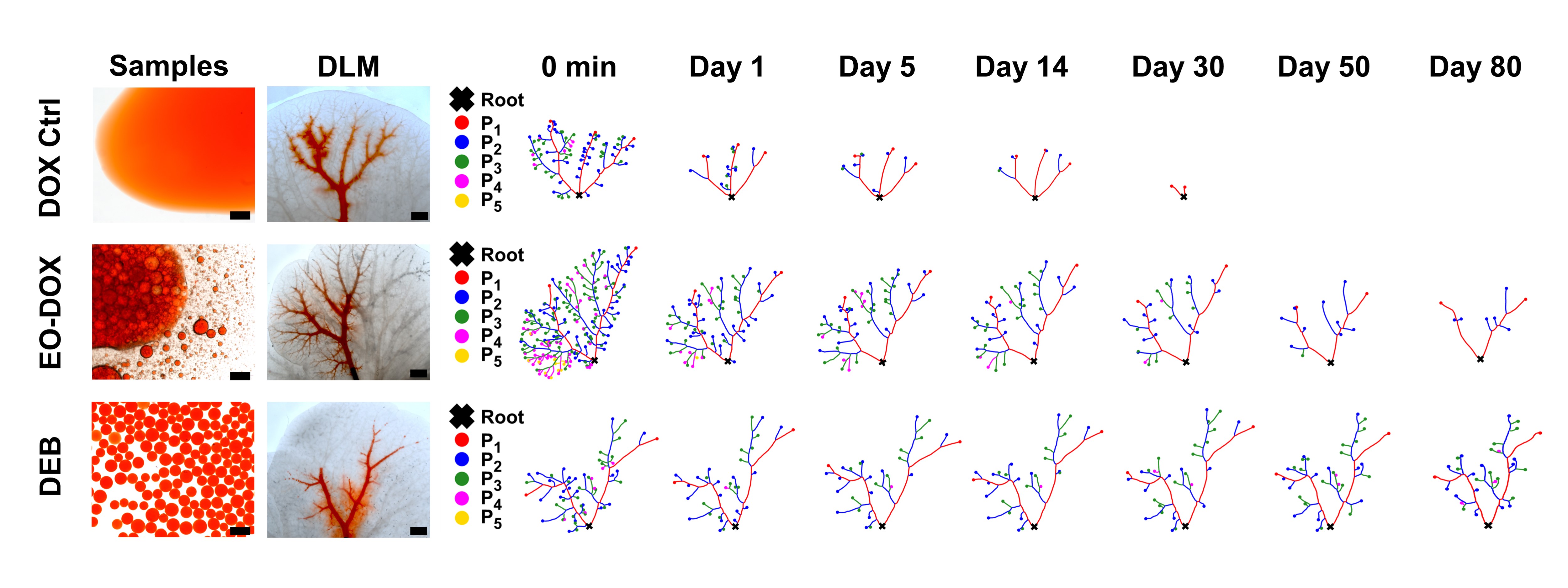
Figure 2 Vessel topological analyses. Skeleton extraction and classification of drug-containing vessels of three drug formulations, i.e., DOX Ctrl, EO-DOX, and DEB, tested in the DLM model over 80 d. DOX: doxorubicin solution; EO-DOX: emulsion of ethiodized oil and doxorubicin solution; DEB: drug eluting bead.
The local drug concentration within tumors is an important factor in determining their therapeutic efficacy. Taking advantage of the visualization capabilities of the 3D model and the strong self-fluorescence of doxorubicin at low concentrations, the researchers determined the extracellular drug diffusion depth as compared to the drug diffusion behavior observed in in vivo experiments. They found that the drug diffusion depth in this 3D model exhibited great linearity with the in-human results, validating the effectiveness of the embolic chemotherapy model for in vivo–in vitro consistency evaluation. This has important guiding significance for the development of new drug formulations and evaluation of embolic chemotherapy efficacy in clinical practice (Figure 3).
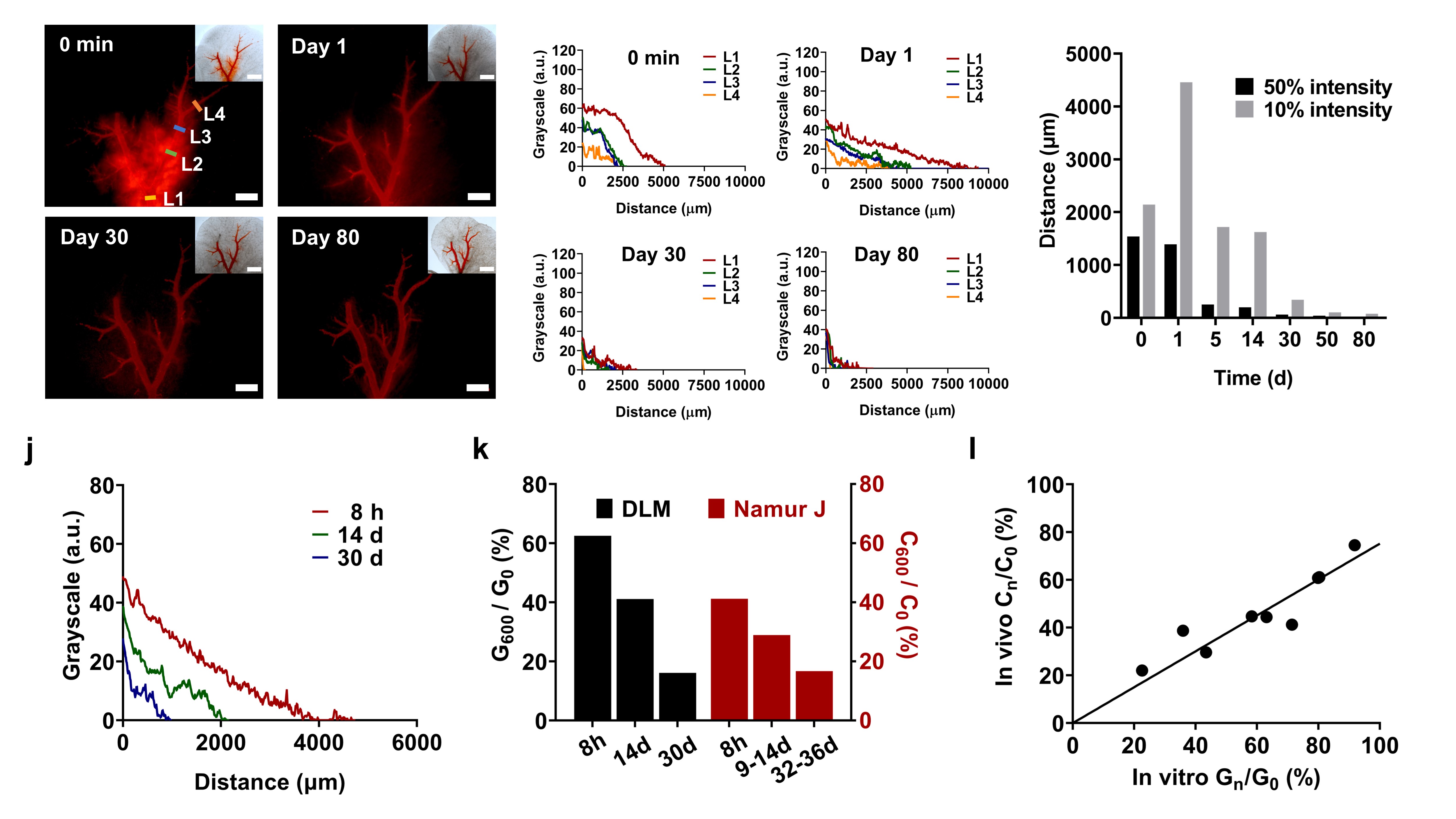
Figure 3 Extravascular drug diffusion of drug eluting beads in DLM model and IVIVC.
Dr. Xiaoya Liu, a postdoctoral fellow from the Department of Biomedical Engineering at SUSTech, and Xueying Wang, a master’s student from the Department of Electronic and Electrical Engineering at SUSTech, are the first authors of this paper. Asst. Prof. Qiongyu Guo and Asst. Prof. Xiaoying Tang are the corresponding authors. SUSTech is the corresponding institution of the paper. Other collaborating authors included master’s students Yucheng Luo, Meijuan Wang, and Xiaoyu Han, all from SUSTech.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Innovative and Entrepreneurial Research Team Program, Science, Technology and Innovation Commission of Shenzhen Municipality, and SUSTech.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/advs.202206195?af=R
Researchers make progress in whole-cell high-throughput 3D super-resolution imaging across large field of view
While fluorescence microscopy with high contrast and super-resolution has revolutionized structural cell biology studies, high-throughput imaging with rich information content has enabled quantitative biology research. An emerging trend is developing high-throughput super-resolution imaging techniques for high-content screening. However, field-dependent aberrations restrict the field of view (FOV) of super-resolution imaging to only tens of micrometers.
Associate Professor Yiming Li from the Advanced Microscopy Imaging Laboratory in the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) led a team to develop a deep learning algorithm framework to address the field-dependent aberrations and bypass the bottleneck for large FOV imaging.
Their research results, entitled “Field-dependent deep learning enables high-throughput whole-cell 3D super-resolution imaging,” has been published in Nature Methods.
The researchers developed a deep learning method for precise localization of spatially variant point emitters (FD-DeepLoc) over a large FOV covering the full chip of a modern sCMOS camera. Using a graphic processing unit (GPU) based vectorial PSF fitter, they can fast and accurately model the spatially variant point spread function (PSF) of a high numerical aperture (NA) objective in the entire FOV. Combined with deformable mirror-based optimal PSF engineering, they demonstrate high-accuracy 3D SMLM over a volume of ~180 × 180 × 5 μm3, allowing people to image mitochondria and nuclear pore complexes in entire cells in a single imaging cycle without hardware scanning – a 100-fold increase in throughput compared to the state-of-the-art.
This work provides new technical ideas and perspectives for the field of super-resolution microscopy. It also has important theoretical and practical value for the study of nanoscale biological structures in complete cell populations or tissues.
.gif)
Figure 1. Large FOV imaging of cellular nuclear pores
Shuang Fu and Wei Shi, doctoral students at SUSTech, are the co-first authors of this paper. Assoc. Prof. Yiming Li is the corresponding author, and SUSTech is the first affiliation.
This work was supported by the Key Technology Research and Development Program of Shandong, Science, Technology and Innovation Commission of Shenzhen Municipality, and Start-Up Fund from SUSTech.
Paper link: https://www.nature.com/articles/s41592-023-01775-5
Prof. Zhi LUO led the National Key R&D Program of SUSTech
The project “Flexible Electronic Materials for Controlled Drug Release and Tissue Regeneration Devices” led by Prof. Zhi LUO, Associate Professor of the Department of Biomedical Engineering, was approved by the Ministry of Science and Technology of China. Born in 1991, Prof. LUO is the youngest chief scientist of the National Key R&D Program of SUSTech.

Researchers make series of advances in field of ionic neuromorphic devices
In nature, the transmission and processing of information, as well as the energy conversation and storage, are often mediated and regulated using ions and fluids transportation at small scales (e.g., nanopores). It can be said that the language of intelligent life is “ions”, and the language of artificial intelligence is “electrons”.
To realize seamless communications between intelligent life and artificial intelligence, building an artificial intelligence system with “ions” as language is necessary. We should fabricate artificial nanopores on the nanoscale to realize controllable ions transport, which is common in the biological nanopore. On a microscale, it is significant to build an artificial neuron to replicate the generation of the action potential by ions transport. On a macroscale, the construction of a bioinspired neural network is necessary to realize ionic signal transmission and information storage.
Associate Professor Kai Xiao’s research team from the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) has made progress on this topic by achieving a series of advances in the field of Bio-inspired Multiscale Ionic Neuromorphic Devices.
Their subsequent papers have been published in top international journals such as Nature Communications, CCS Chemistry, Advanced Science, and ACS Nano.
Ionic diode property widely exists in the biological nanopore of intelligent living organisms. To control ions transport unidirectional, cells living organisms can realize a series of physiological activities such as the generation of action potentials and the control of cell osmotic pressure.
To realize ionic diode properties in vitro, Prof. Xiao’s team fabricated a carbon-based nanofluid with multiple scales and realized ionic diode properties with an ionic rectification ratio (on-off ratio) of more than 10,000. This work breaks the existing diode mode of “silicon materials + electrons”, and builds a life-like new diode model of “carbon materials + ions”. This lays a good foundation for the construction of ion transport-based logic circuits and derives ion transport-based transistors and neurons.
The related research results, entitled “Unidirectional ion transport in nanoporous carbon membranes with a hierarchical pore architecture,” were published in Nature Communications.
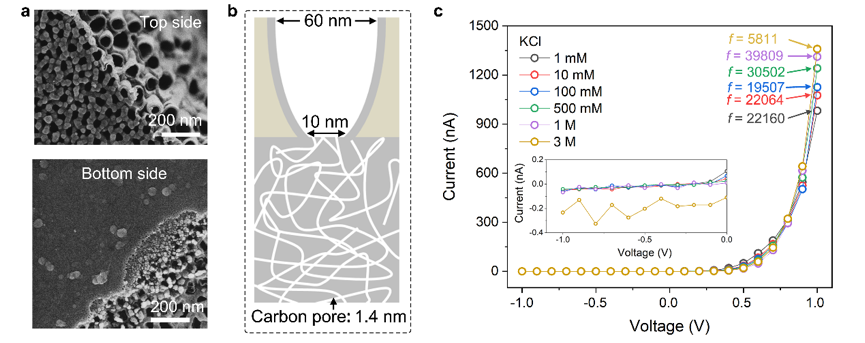
Figure 1. Carbon-based nanofluids with multiple scales and its ionic diode properties
An ion pump is a unique function of intelligent life. It can realize the reverse concentration gradient transport of ions by consuming external energy. This progress is important for many physiological activities, such as photosynthesis, ATP synthesis, and action potential generation. How to construct a biomimetic ion pump can lay a good foundation for the technological breakthrough of various devices such as ion transport-based photoelectric energy conversion and neural signal regulation.
In their earlier research (Nat. Commun. 2019, 10, 74; Natl. Sci. Rev. 2021, 8, nwaa231.), the researchers realized a series of biomimetic ion pump functions and applications by constructing a nanofluidic system based on semiconductor materials. Most recently, they proposed that biomimetic ion pumps can be constructed by introducing asymmetric elements into a nanofluids system. For example, for the widely studied biomimetic light-driven ion pumps, light-driven ion pump properties can be realized by the asymmetric photoelectric effect, photothermal effect, and photochemical reaction, respectively
This study, entitled “Light-Driven Ion Transport in Nanofluidic Devices: Photochemical, Photoelectric, and Photothermal Effects,” was published in CCS Chemistry.
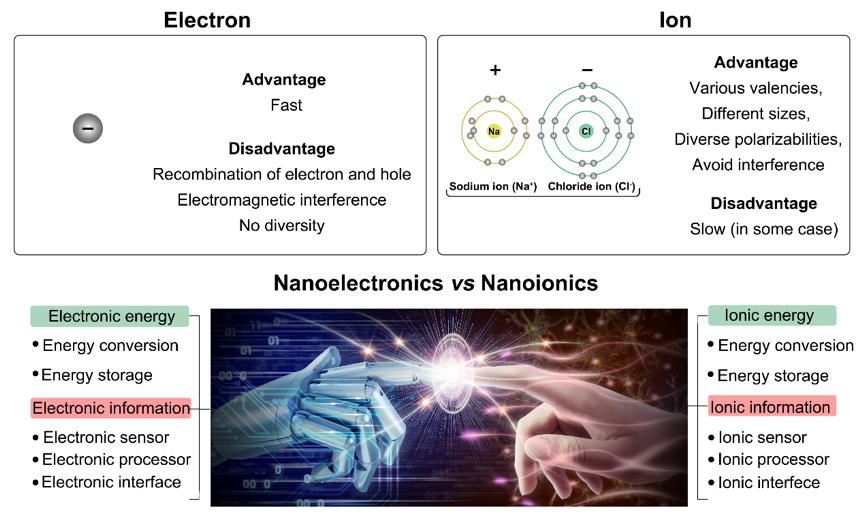
Figure 2. Compared to electrons, ions have many unique advantages in designing energy and information devices. For example, various valencies, different sizes, and diverse polarizabilities. These characterizations enable all energy and information processors constructed by electrons to be redefined by ions.
In recent years, with the development of artificial intelligence (AI) and a deeper understanding of biological intelligence, researchers have found that devices based on electron transport have significant limitations in the process of realizing human-computer interaction. By learning from nature, constructing ions transport-based energy and sensor devices can lay a good foundation for developing the next-generation brain-computer interface and realizing seamless human-computer interaction. This research topic involves interdisciplinary fields such as chemistry, materials, devices, and biology.
Prof. Xiao’s research group was invited to publish a series of reviews and perspectives on how to construct nanoionic devices to realize the functions that nanoelectronic devices cannot satisfy.
These reviews, entitled “Nanoionics from Biological to Artificial Systems: An Alternative Beyond Nanoelectronics” and “Solid-State Iontronic Devices: Mechanisms and Applications,” were published in Advanced Science and Advanced Materials Technologies, respectively.
They pointed out that ions can realize many of the functions that nanoelectronics can achieve. By learning from intelligent life, nanoionics devices also can realize some functions that nanoelectronics cannot. Meanwhile, these perspective articles analyze and compare the advantages and disadvantages of various devices based on ions and electrons. Based on ion transport, they predict that biomimetic nanoionic devices will be another research hotspot following nanoelectronic devices.
Dr. Jianrui Zhang, Dr. Tianming Li, Ms. Tingting Mei, and Ms. Hongjie Zhang are the first authors of the above papers and reviews. Assoc. Prof. Kai Xiao is the corresponding author, while SUSTech is the first affiliation. This work was supported by the National Key Research of China.
Paper and related links (In order of appearance above):
Nature Communications: https://www.nature.com/articles/s41467-021-24947-3
CCS Chemistry: https://www.chinesechemsoc.org/doi/full/10.31635/ccschem.021.202101297
Advanced Science: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.202200534
Advanced Materials Technologies: https://onlinelibrary.wiley.com/doi/10.1002/admt.202200205
Prof. Kai Xiao’s group webpage: http://www.xiaokai-group.cn/
SUSTech students win gold award in China’s first synthetic biology competition
A team of students from the Southern University of Science and Technology (SUSTech) recently won the Gold Award and the Best Website Award in China’s first synthetic biology innovation competition, SynBio Challenges 2022.
The competition was sponsored by the Synthetic Biology Branch of the Chinese Society of Bioengineering, which aims to provide a platform for young students to communicate, learn, innovate, create, and cultivate their knowledge in synthetic biology, life sciences, and interdisciplinary disciplines.
Twenty-seven teams from 21 universities and colleges across the country participated in SynBio Challenges 2022, and more than 2.2 million people viewed the event online.

The SUSTech team, titled “SUSTech_Shenzhen_HCL”, was led by Prof. Ho Chun Loong from the Department of Biomedical Engineering as the Principal Investigator, Jun CHEN and Xinyi CHEN as the instructors, and Liqing YU, Songlin SHI, and Yehan WANG as the team leaders.
In addition, 14 members from different majors, including Yingxuan GONG, Xianxian WANG, Yijie WANG, Siqi MEN, Hongqiu LEI, Jiayi LIU, Nanfei JIANG, and Xuancheng MO, formed the multidisciplinary team.
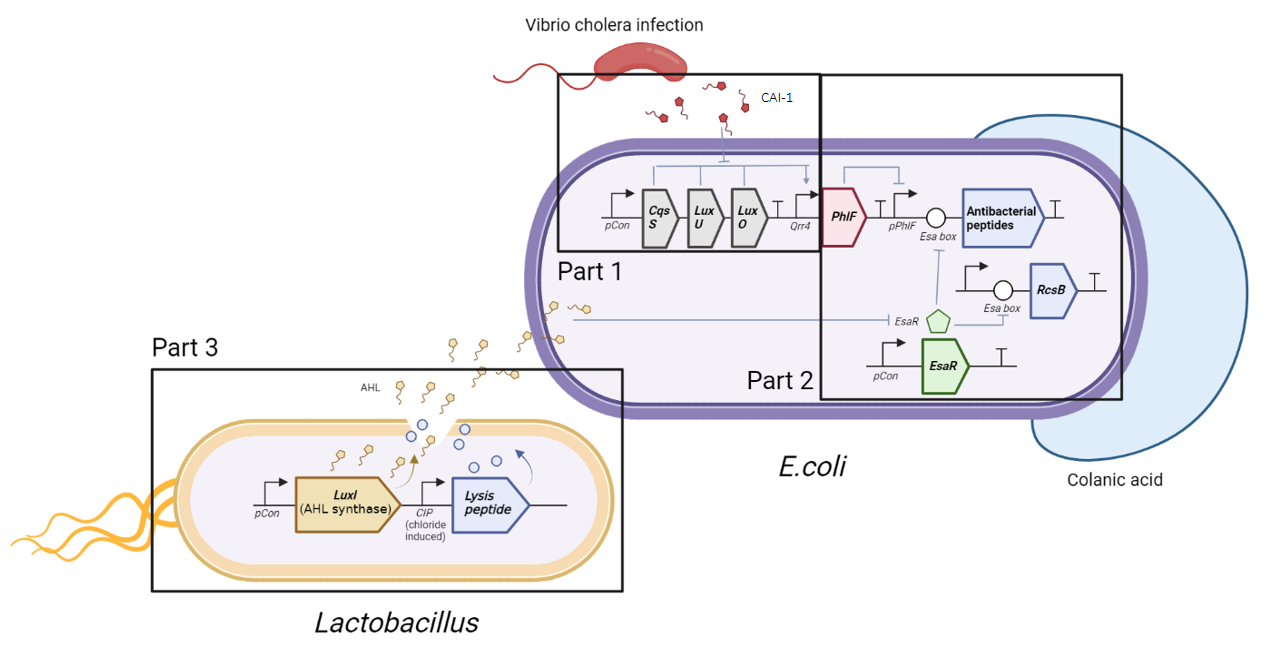
Cholera is an acute diarrheal illness caused by infection of the intestine with Vibrio cholerae bacteria, which can cause dehydration and death in severe cases.
The illness is associated with dysregulation of the gut microbiota, so modulation of the gut microbiota could be a treatment for cholera.
Through genetic engineering technology, the SUSTech_Shenzhen_HCL team used Lactobacillus and Escherichia coli (E. coli) bacteria as carriers. They developed a probiotic system that can prevent and treat through the group effect between bacteria. As a result, the team won the Gold Award and the Best Website Award.
SUSTech hosts 10th WACBE World Congress on Bioengineering
On April 22-23, 2022, the World Association for Chinese Biomedical Engineers (WACBE) held its 10th World Congress on Bioengineering. The virtual event was hosted by the Department of Biomedical Engineering (BME) at the Southern University of Science and Technology (SUSTech).

Many experts and scholars presented plenary and keynote reports during the congress, which attracted many young scholars from different disciplinary and research backgrounds to participate in the event.
The conference promotes the exchange of the latest research results in the field of biomedical engineering and enhances the intersection and integration of related disciplines.
The WACBE World Congress on Bioengineering is an annual event that focuses on biomedical engineering approaches that drive innovative technologies and foster solutions. The congress attracts about 400 delegates from all over the world, including academic researchers, industry leaders, medical experts, and trainees.
The 10th WACBE World Congress on Bioengineering
Conference Information
The 10th WACBE World Congress in Bioengineering is taking place at Southern University of Science and Technology, Shenzhen, China from April 22 to 23, 2022.
The WACBE World Congress on Bioengineering focuses on biomedical engineering approaches that drive innovative technologies and foster solutions that directly impact clinical medical practice. The Congress offers a platform, that the attendees can collaborate on the clinical translation of biomedical technologies. The Congress attracts about 400 delegates from all over the world, including academic researchers, industry leaders, medical experts, and trainees. They are gathering in the Congress to share their experience on bioengineering and showcase contributions that biomedical engineers bring to the health care sector, with new applications that are vital to the quality of life.
Due to COVID-19 restrictions, the conference will switch to the online only format, using VOOV (international version of Tencent Meeting).
Conference website: https://www.wacbe2022.com/