Researchers from Southern University of Science and Technology (SUSTech) and Fuwai Hospital of the Chinese Academy of Medical Sciences ( referred to as “Fuwai Hospital”) have developed electronic blood vessels that can be actively tuned to address subtle changes in the body after implantation. The research, published in the journal Matter, entitled “Electronic Blood Vessel.”
By using poly(L-lactide-co-ε-caprolactone) (PLC), the electronic blood vessels are made to encapsulate liquid metal to achieve the flexible and biodegradable circuit. It could also overcome the limitations of conventional tissue-engineered blood vessels (TEBVs), which serve as passive scaffolds., This could be achieved by coordinating with other electronic devices to deliver genetic material, enable controlled drug release, and facilitate the formation of new endothelial blood vessel tissue.
The electronic blood vessels could integrate flexible electrons with three layers of blood vessel cells to imitate and surpass natural blood vessels. It can effectively promote cell proliferation and migration in the wound healing model through electrical stimulation. It can controllably deliver genes to specific parts of the blood vessel through electro-transfection. Through a 3-month in vivo study of the rabbit carotid artery replacement model, the authors evaluated the electronic blood vessel’s efficacy and biological safety in the vascular system. They confirmed its patency through ultrasound imaging and angiography. The research paves the way for the integration of flexible, degradable bioelectronics into the vascular system, which can be used as a platform for further treatments, such as gene therapy, electrical stimulation, and electronically controlled drug release.
Electronic blood vessels show the potential to play a key role in the treatment of cardiovascular disease. In the treatment of cardiovascular disease through coronary artery bypass grafting, the existing small diameter (<6 mm) tissue-engineered blood vessels (TEBV) have not yet met clinical needs. The methods used in most studies only provide TEBV with a stent that provides mechanical support, which mainly relies on the remodeling process of host tissues but has obvious limitations in helping to regenerate new blood vessels. Specifically, the complex interaction between blood flow and TEBV usually causes resonance reactions, leading to problems such as thrombus formation and neointimal hyperplasia. Instead, the new generation of TEBV should have enough apical stents to provide mechanical support and the ability to respond and integrate with the remodeling process actively to provide adaptive treatment after implantation.
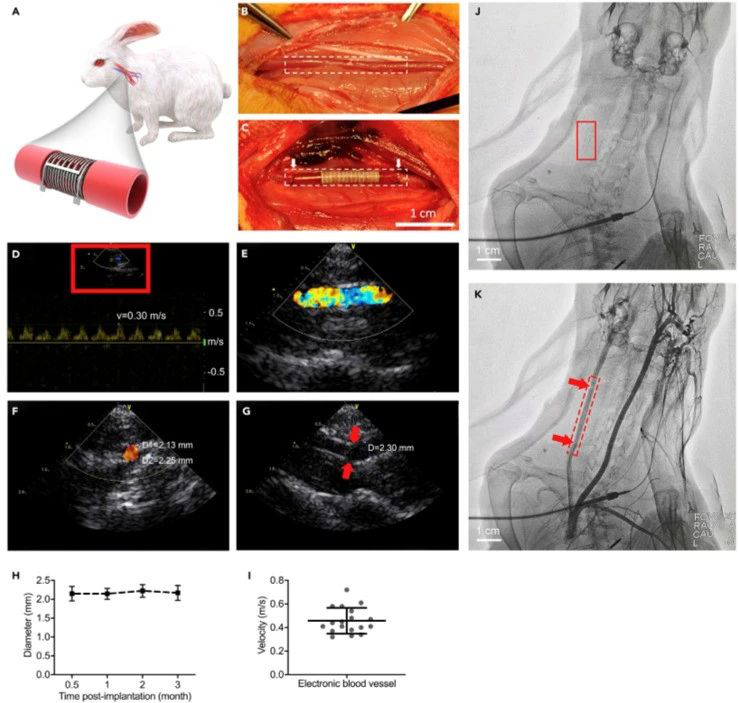
In Situ Monitoring of the Electronic Blood Vessel
(A) Schematic of the electronic blood vessel in the carotid artery of the rabbit.
(B and C) An end-to-end anastomosis procedure of electronic blood vessel implantation in carotid arteries of rabbits (n = 6). The dotted frames (B and C)outline the margin of the native carotid artery and the implanted electronic blood vessel. The white arrows (C) indicate the two ends of the electronic blood vessel. Scale bar, 1 cm.
(D–G) In situmonitoring of the electronic blood vessel by Doppler ultrasound imaging 3 months post-implantation. Representative images from at least three different animals. (D and E) The real-time blood flow at the operational site and the synchronized ultrasound pulses. The asymmetric velocity curve indicates that the signal is from the carotid artery rather than the vein. (E) Zoom-in view of red box in (D). (F) The cross-sectional view of the blood flow.
(G) The suture site (red arrows) connecting the native carotid artery and the electronic blood vessel.
(H) The diameter changes in the electronic blood vessel at different times post-implantation.
(I) The velocity of blood flow at the operational site (n = 18, from different rabbits at different time points).
(J and K) In situ monitoring by arteriography 3months post-implantation. (J) Image before injecting the contrastmedium. Red box indicates the position of the implanted electronic blood vessel. Scale bar, 1 cm.
(K) Image after injecting the contrast medium. Red box indicates the position of the implanted blood vessel. Red arrows indicate the suture sites connecting the native carotid artery and the electronic blood vessel. Scale bar, 1 cm.
The team uses a PTFE mandrel, forming a 3D multi-layered tubular structure with PLC-based metal-polymer conductor (MPC) membranes. The inner diameter of the electronic blood vessel is about 2 mm, which is flexible and degradable. The MPC circuit has excellent conductivity and can be well distributed in the three-dimensional multilayer tubular structure. Studies have found that electronic blood vessels also have excellent cell safety, including the three cultured vascular cells (human umbilical vein endothelial cells, human aortic smooth muscle cells, and human aortic fibroblasts).
The team also constructed a 3D electrical function model to stimulate endothelial cells in vitro through electrochemical workstations to promote their proliferation and migration. Simultaneously, three kinds of blood vessel cells were patterned on the 3D electronic blood vessel, and the GFP plasmid was electroporated in vitro by an electroporation instrument. After two days of culture, the expression of the plasmid was observed.
The team chose rabbits from New Zealand as the animal model, replacing the common carotid artery with electronic blood vessels. The implanted electronic blood vessels were monitored by Doppler ultrasound imaging and arteriography. According to Doppler ultrasound imaging, three months after implantation, the electronic blood vessel allows stable blood flow to pass, showing the electronic blood vessel’s excellent patency. In the future, the electronic blood vessel can be integrated with other electronic components and devices to achieve diagnostic and therapeutic functions. This will enhance significantly personalized medical functions by establishing a direct connection in the blood vessel tissue-machine interface.
Shiyu Cheng, Chen Hang, and Li Ding are the joint first authors of the paper, and Yan Zhang (Fuwai Hospital) and Xingyu Jiang (SUSTech)are the corresponding authors.
Link to the paper: https://www.sciencedirect.com/science/article/pii/S2590238520304938